Atomic structure whats in it for plasmas Yuri




- Slides: 45

Atomic structure: what’s in it for plasmas? . . Yuri Ralchenko National Institute of Standards and Technology Gaithersburg, MD 20899, USA

Why is atomic structure important for plasmas? Most of the relevant physics is inside this matrix element final state • • • interaction operator initial state Wavelengths Energies Transition probabilities (radiative and non-radiative) Collisional cross sections …

A Few Textbooks on APP ● H. R. Griem – – ● R. D. Cowan – ● Theory of Atomic Structure and Spectra (1981) V. P. Shevelko and L. A. Vainshtein – ● Plasma Spectroscopy (1964) Principles of Plasma Spectroscopy (1997) Atomic Physics for Hot Plasmas (1993) D. Salzmann – Atomic Physics in Hot Plasmas (1998) • T. Fujimoto • Plasma Spectroscopy (2004) • H. -J. Kunze • Introduction to Plasma Spectroscopy (2009) • J. Bauche, C. Bauche. Arnoult, O. Peyrusse • Atomic Properties in Hot Plasmas (2015) • Modern Methods in Collisional-Radiative Modeling of Plasmas (2016) • HKC, HAS, YR, …

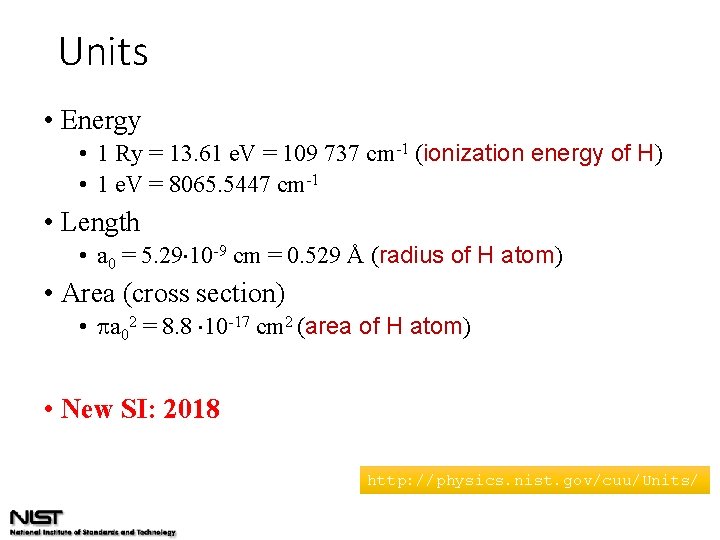
Units • Energy • 1 Ry = 13. 61 e. V = 109 737 cm-1 (ionization energy of H) • 1 e. V = 8065. 5447 cm-1 • Length • a 0 = 5. 29 10 -9 cm = 0. 529 Å (radius of H atom) • Area (cross section) • a 02 = 8. 8 10 -17 cm 2 (area of H atom) • New SI: 2018 http: //physics. nist. gov/cuu/Units/

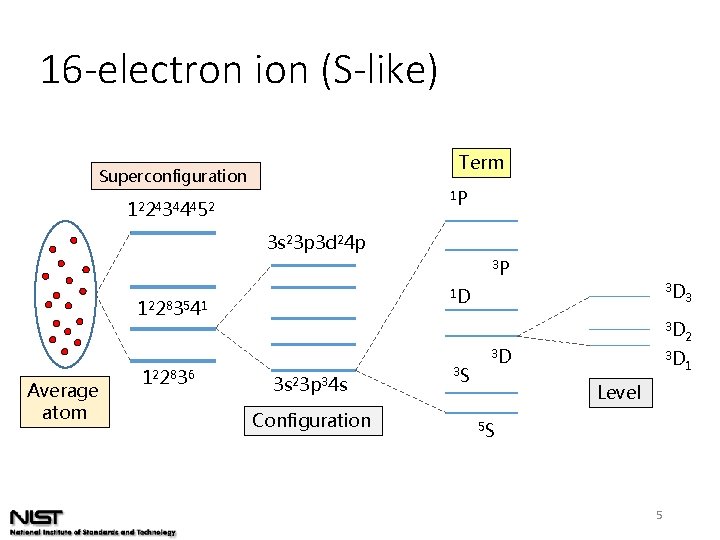
16 -electron ion (S-like) Term Superconfiguration 1 P 12 24 34 44 52 3 s 23 p 3 d 24 p 3 P 1 D 12 28 35 41 Average atom 12 28 36 3 s 23 p 34 s Configuration 3 S 3 D Level 5 S 5 3 D 3 3 D 2 3 D 1

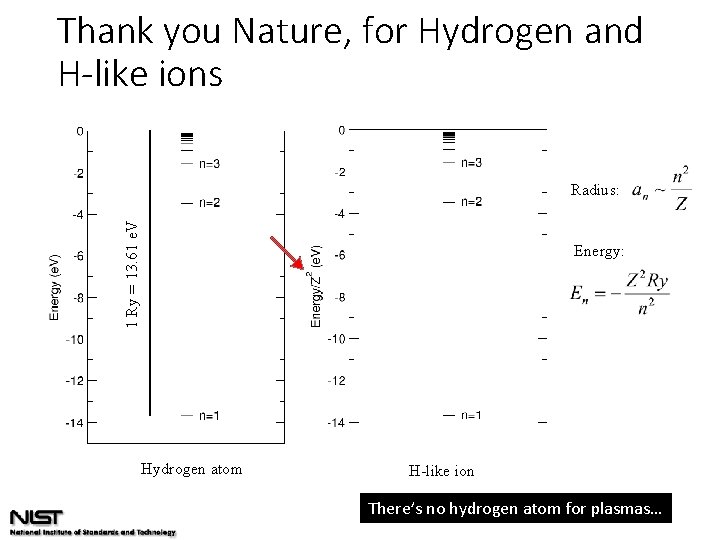
Thank you Nature, for Hydrogen and H-like ions 1 Ry = 13. 61 e. V Radius: Energy: Hydrogen atom H-like ion There’s no hydrogen atom for plasmas…

Exact quantum numbers for general atomic states •

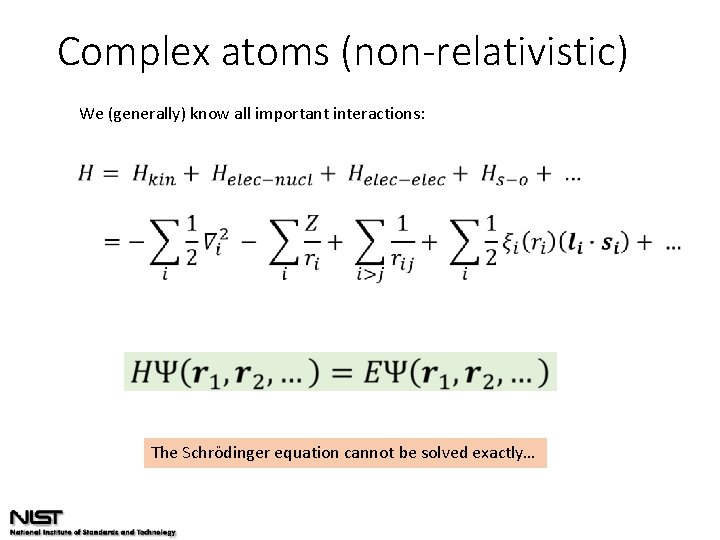
Complex atoms (non-relativistic) We (generally) know all important interactions: The Schrödinger equation cannot be solved exactly…

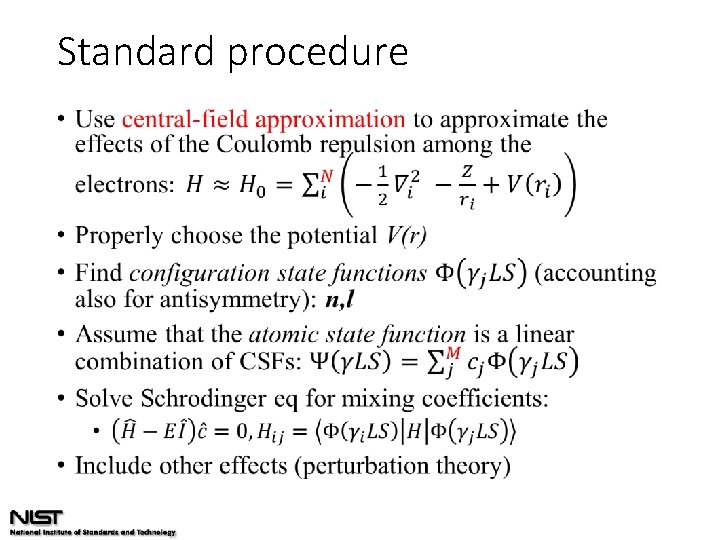
Standard procedure •

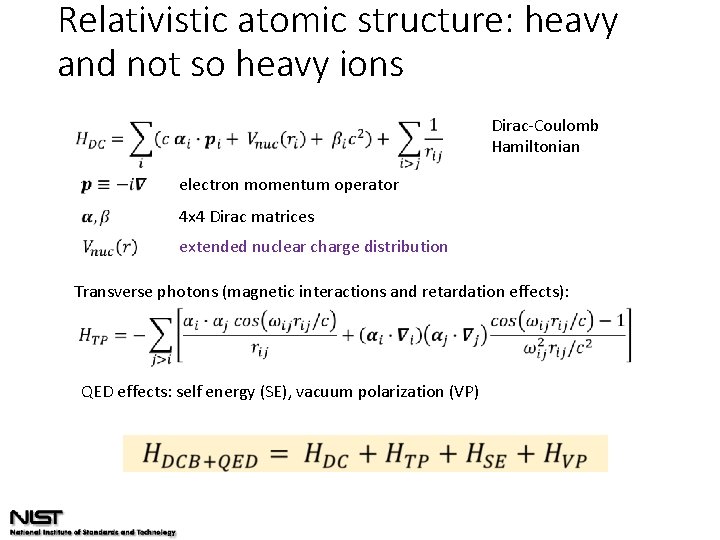
Relativistic atomic structure: heavy and not so heavy ions Dirac-Coulomb Hamiltonian electron momentum operator 4 x 4 Dirac matrices extended nuclear charge distribution Transverse photons (magnetic interactions and retardation effects): QED effects: self energy (SE), vacuum polarization (VP)

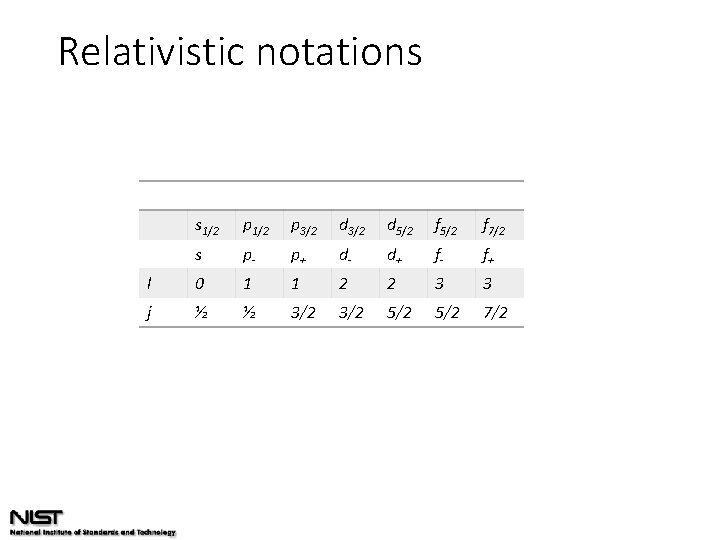
Relativistic notations s 1/2 p 3/2 d 5/2 f 7/2 s p- p+ d- d+ f- f+ l 0 1 1 2 2 3 3 j ½ ½ 3/2 5/2 7/2

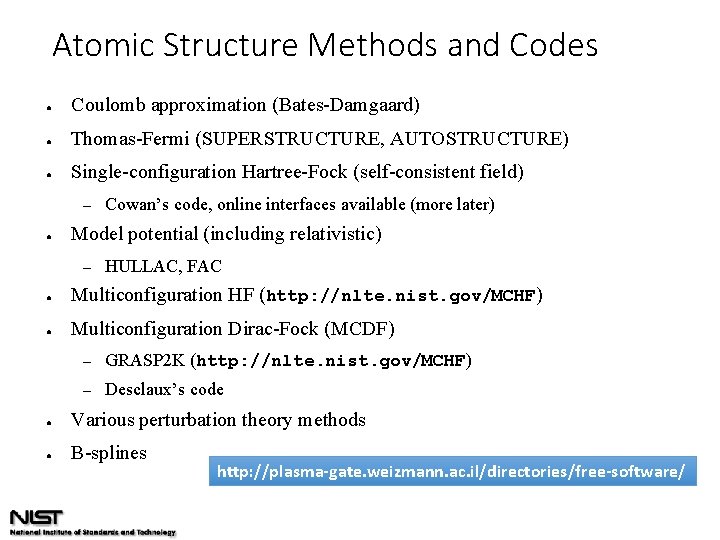
Atomic Structure Methods and Codes ● Coulomb approximation (Bates-Damgaard) ● Thomas-Fermi (SUPERSTRUCTURE, AUTOSTRUCTURE) ● Single-configuration Hartree-Fock (self-consistent field) – ● Cowan’s code, online interfaces available (more later) Model potential (including relativistic) – HULLAC, FAC ● Multiconfiguration HF (http: //nlte. nist. gov/MCHF) ● Multiconfiguration Dirac-Fock (MCDF) – GRASP 2 K (http: //nlte. nist. gov/MCHF) – Desclaux’s code ● Various perturbation theory methods ● B-splines http: //plasma-gate. weizmann. ac. il/directories/free-software/

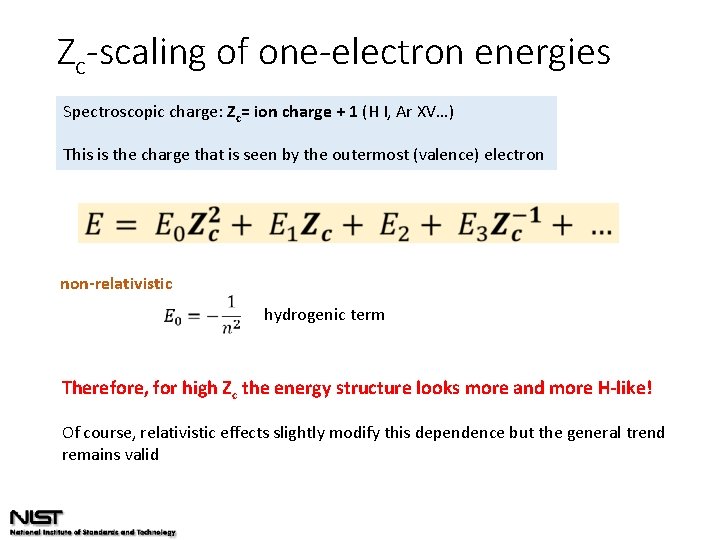
Zc-scaling of one-electron energies Spectroscopic charge: Zc= ion charge + 1 (H I, Ar XV…) This is the charge that is seen by the outermost (valence) electron non-relativistic hydrogenic term Therefore, for high Zc the energy structure looks more and more H-like! Of course, relativistic effects slightly modify this dependence but the general trend remains valid

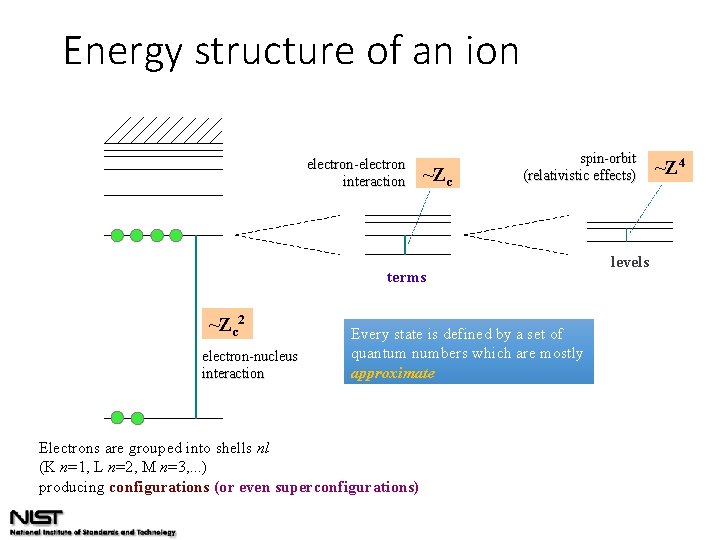
Energy structure of an ion electron-electron interaction ~Zc spin-orbit (relativistic effects) terms ~Zc 2 electron-nucleus interaction Every state is defined by a set of quantum numbers which are mostly approximate Electrons are grouped into shells nl (K n=1, L n=2, M n=3, . . . ) producing configurations (or even superconfigurations) levels ~Z 4

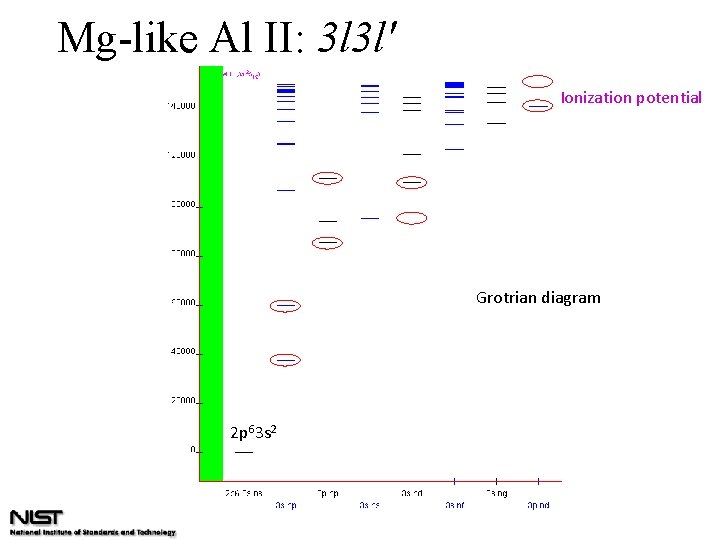
Mg-like Al II: 3 l 3 l' Ionization potential Grotrian diagram 2 p 63 s 2

Mg-like Sr XXVII: 3 l 3 l' Ionization potential 2 p 63 s 2

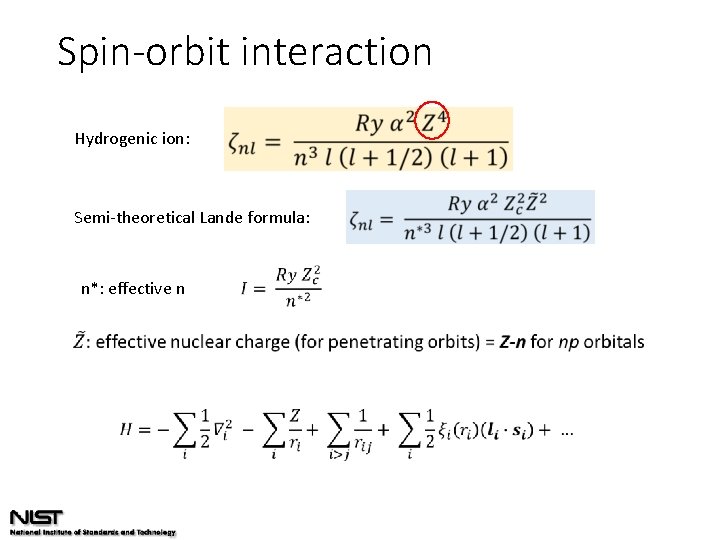
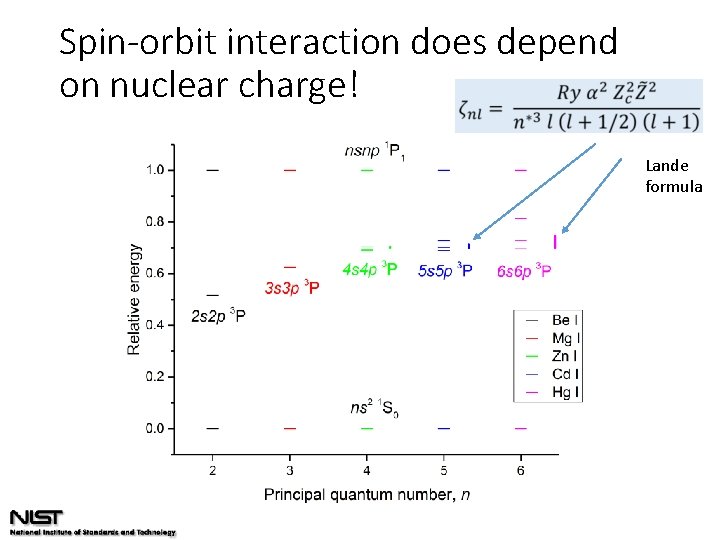
Spin-orbit interaction Hydrogenic ion: Semi-theoretical Lande formula: n*: effective n

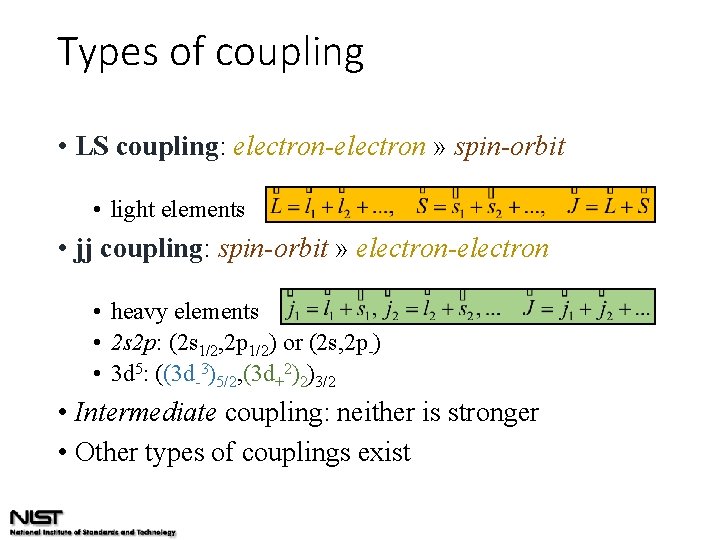
Types of coupling • LS coupling: electron-electron » spin-orbit • light elements • jj coupling: spin-orbit » electron-electron • heavy elements • 2 s 2 p: (2 s 1/2, 2 p 1/2) or (2 s, 2 p-) • 3 d 5: ((3 d-3)5/2, (3 d+2)2)3/2 • Intermediate coupling: neither is stronger • Other types of couplings exist

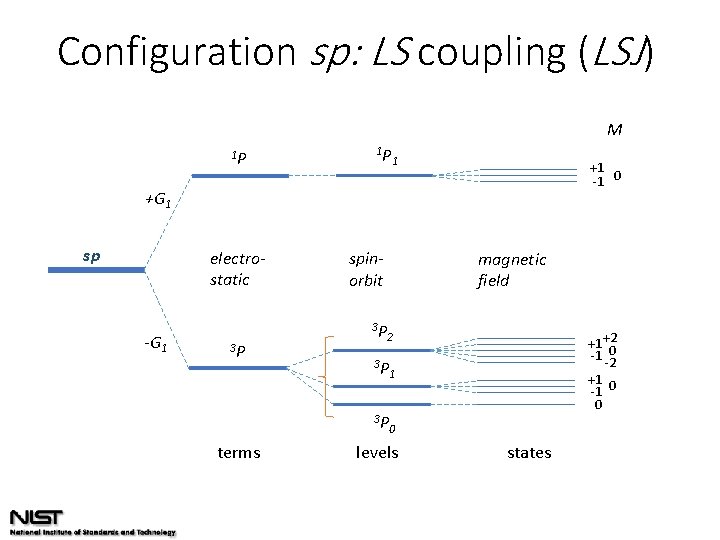
Configuration sp: LS coupling (LSJ) M 1 P 1 P 1 +1 -1 0 +G 1 sp electrostatic -G 1 3 P terms spinorbit 3 P magnetic field +1 +2 -1 0 -2 +1 0 -1 0 2 3 P 1 3 P 0 levels states

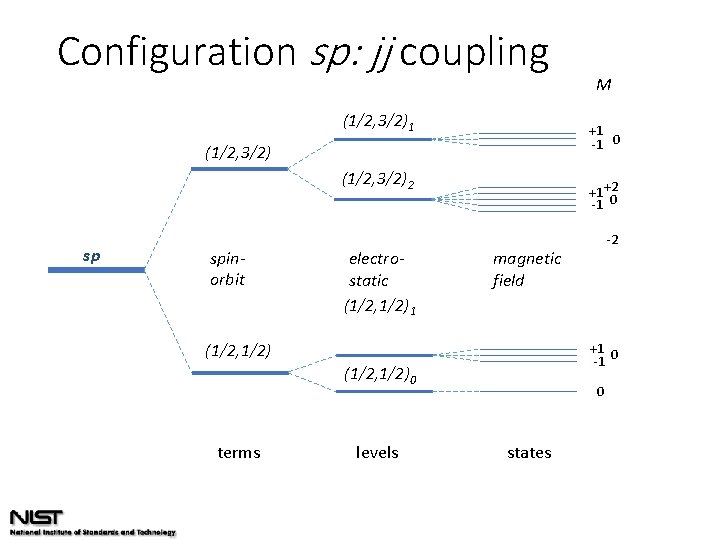
Configuration sp: jj coupling (1/2, 3/2)1 +1 -1 0 (1/2, 3/2)2 sp spinorbit electrostatic (1/2, 1/2)1 +1 +2 -1 0 +1 0 -1 (1/2, 1/2)0 levels -2 magnetic field (1/2, 1/2) terms M 0 states

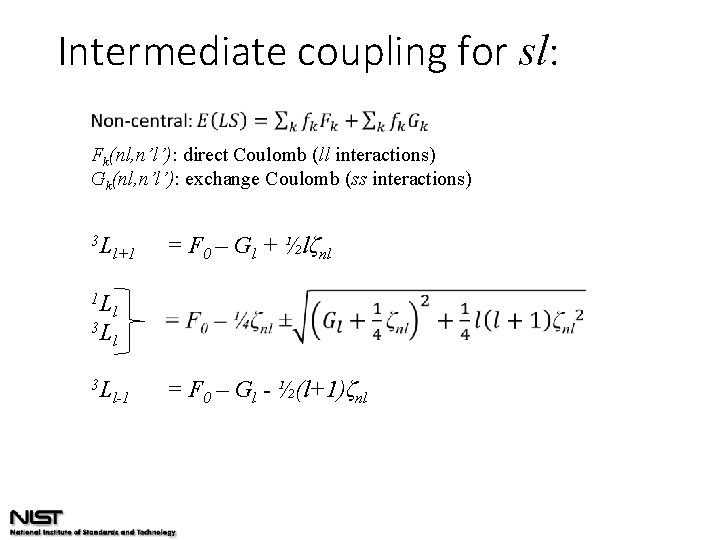
Intermediate coupling for sl: Fk(nl, n’l’): direct Coulomb (ll interactions) Gk(nl, n’l’): exchange Coulomb (ss interactions) 3 L 1 L 3 L 3 L l+1 l = F 0 – Gl + ½lζnl l l-1 = F 0 – Gl - ½(l+1)ζnl

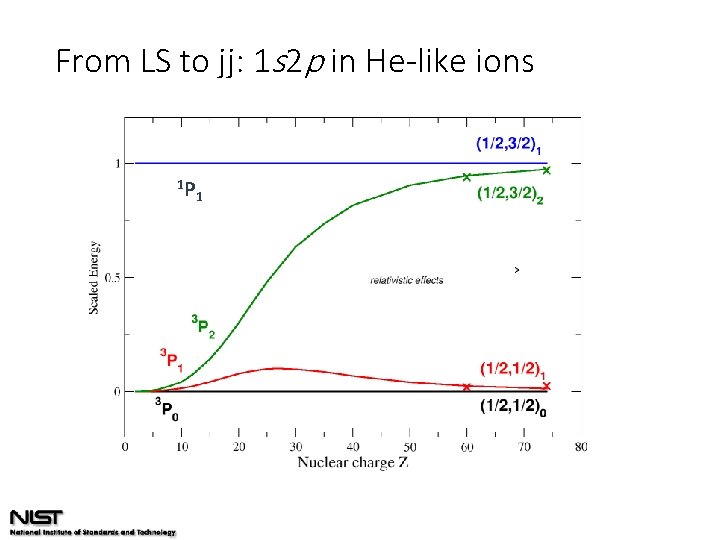
From LS to jj: 1 s 2 p in He-like ions 1 P 1

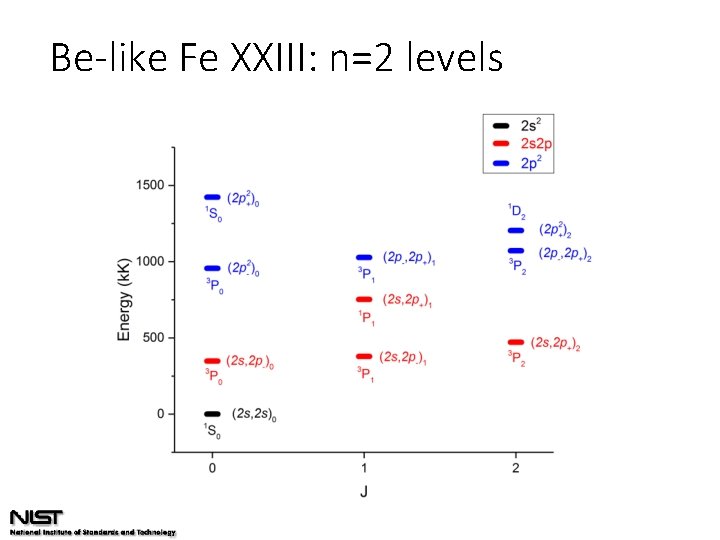
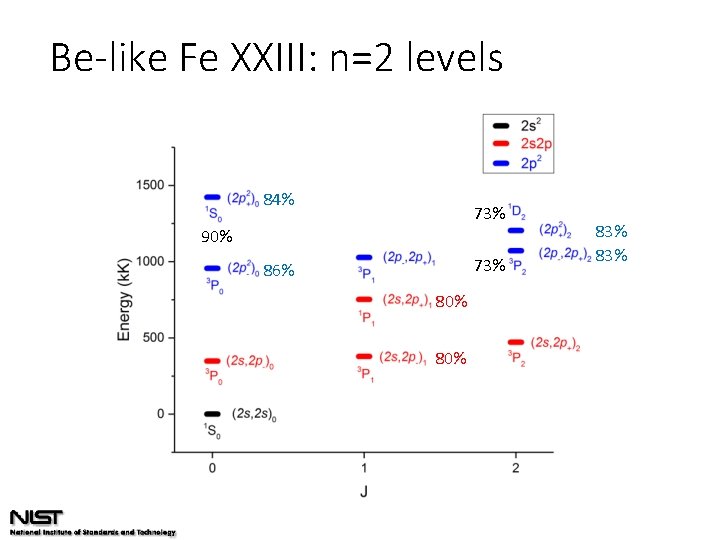
Be-like Fe XXIII: n=2 levels

Be-like Fe XXIII: n=2 levels 84% 73% 90% 73% 86% 80% 83%

Spin-orbit interaction does depend on nuclear charge! Lande formula

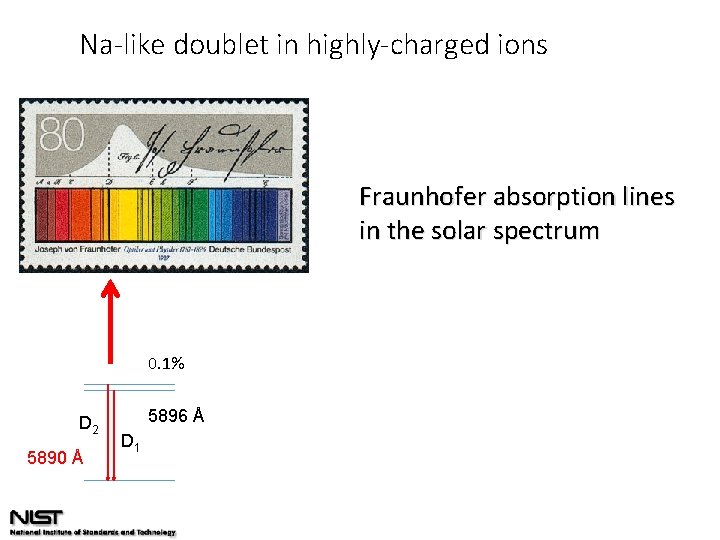
Na-like doublet in highly-charged ions Fraunhofer absorption lines in the solar spectrum 0. 1% D 2 5890 Å 5896 Å D 1

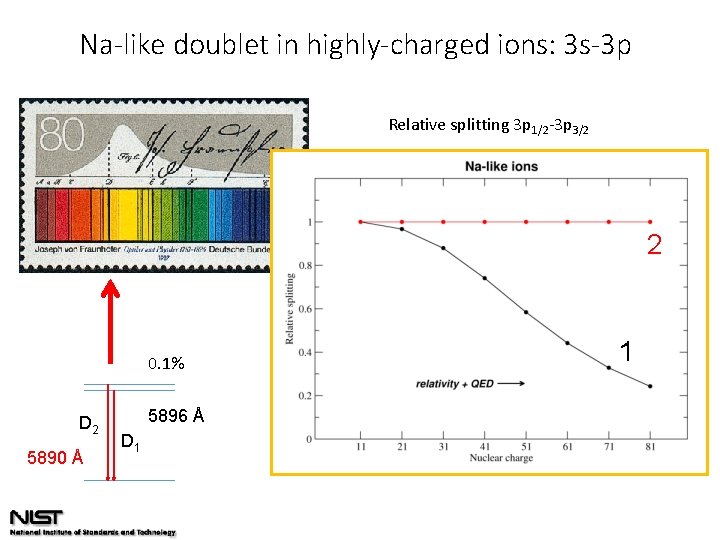
Na-like doublet in highly-charged ions: 3 s-3 p Relative splitting 3 p 1/2 -3 p 3/2 2 0. 1% D 2 5890 Å 5896 Å D 1 1

Little Ions With a Big Charge Sodium Atom 11 electrons Sodium-like Tungsten (W 63+)

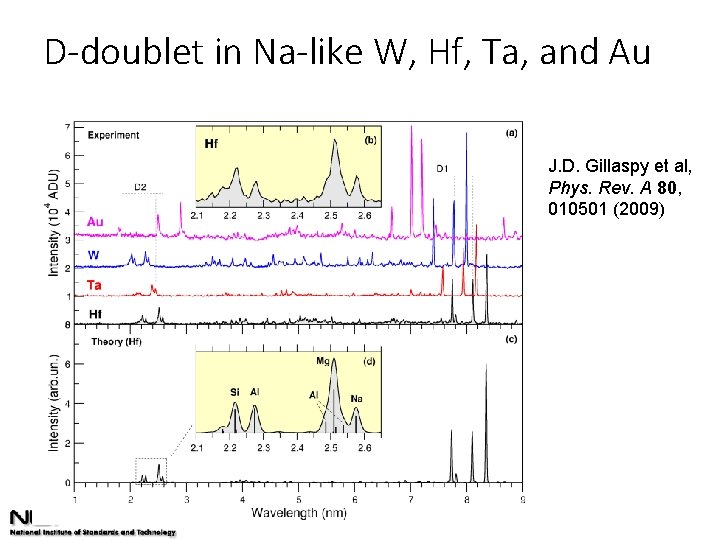
D-doublet in Na-like W, Hf, Ta, and Au J. D. Gillaspy et al, Phys. Rev. A 80, 010501 (2009)

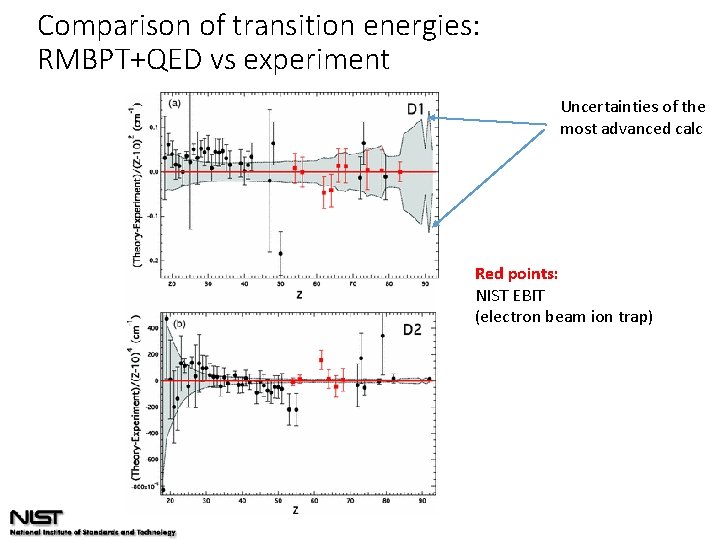
Comparison of transition energies: RMBPT+QED vs experiment Uncertainties of the most advanced calc Red points: NIST EBIT (electron beam ion trap)

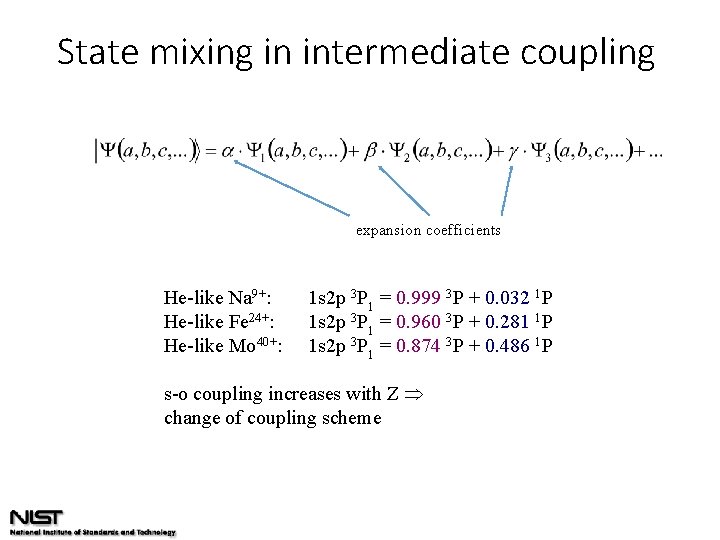
State mixing in intermediate coupling expansion coefficients He-like Na 9+: He-like Fe 24+: He-like Mo 40+: 1 s 2 p 3 P 1 = 0. 999 3 P + 0. 032 1 P 1 s 2 p 3 P 1 = 0. 960 3 P + 0. 281 1 P 1 s 2 p 3 P 1 = 0. 874 3 P + 0. 486 1 P s-o coupling increases with Z change of coupling scheme

Other types of coupling •

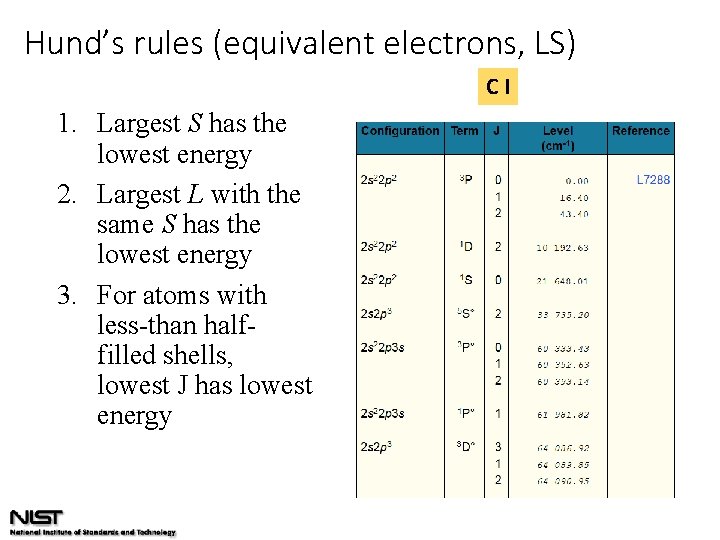
Hund’s rules (equivalent electrons, LS) CI 1. Largest S has the lowest energy 2. Largest L with the same S has the lowest energy 3. For atoms with less-than halffilled shells, lowest J has lowest energy

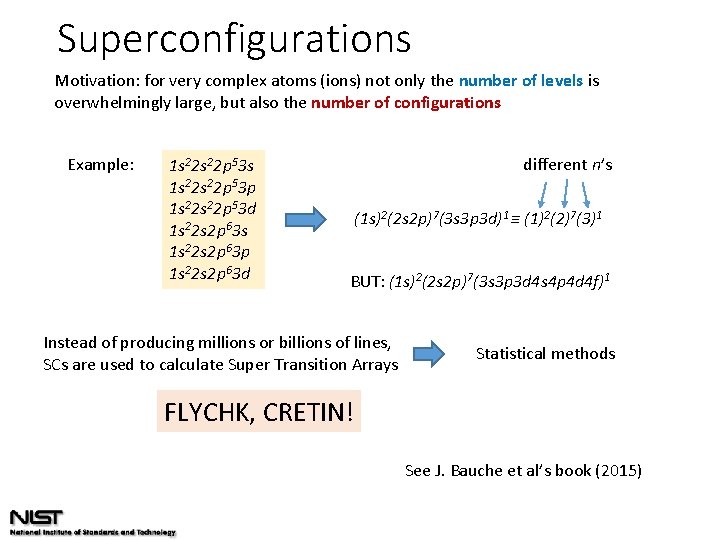
Superconfigurations Motivation: for very complex atoms (ions) not only the number of levels is overwhelmingly large, but also the number of configurations Example: 1 s 22 p 53 s 1 s 22 p 53 p 1 s 22 p 53 d 1 s 22 s 2 p 63 s 1 s 22 s 2 p 63 p 1 s 22 s 2 p 63 d different n’s (1 s)2(2 s 2 p)7(3 s 3 p 3 d)1 ≡ (1)2(2)7(3)1 BUT: (1 s)2(2 s 2 p)7(3 s 3 p 3 d 4 s 4 p 4 d 4 f)1 Instead of producing millions or billions of lines, SCs are used to calculate Super Transition Arrays Statistical methods FLYCHK, CRETIN! See J. Bauche et al’s book (2015)

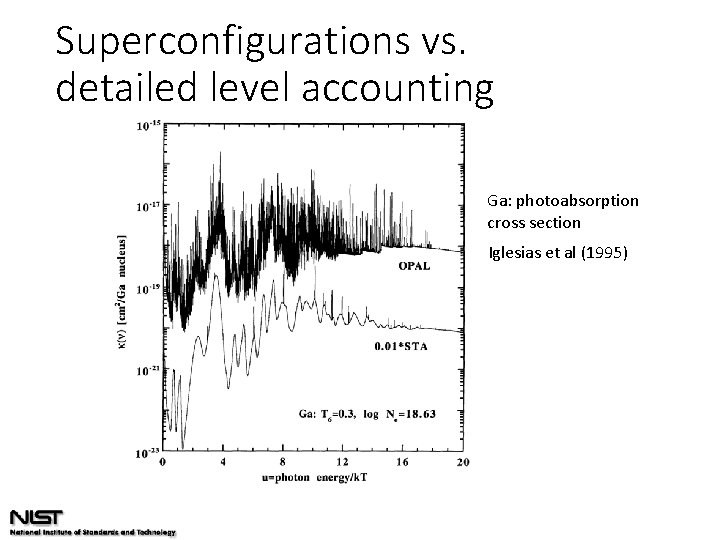
Superconfigurations vs. detailed level accounting Ga: photoabsorption cross section Iglesias et al (1995)

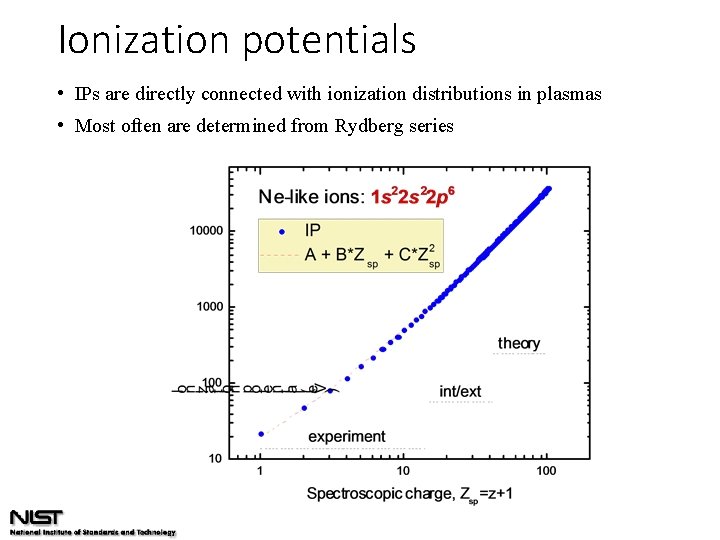
Ionization potentials • IPs are directly connected with ionization distributions in plasmas • Most often are determined from Rydberg series

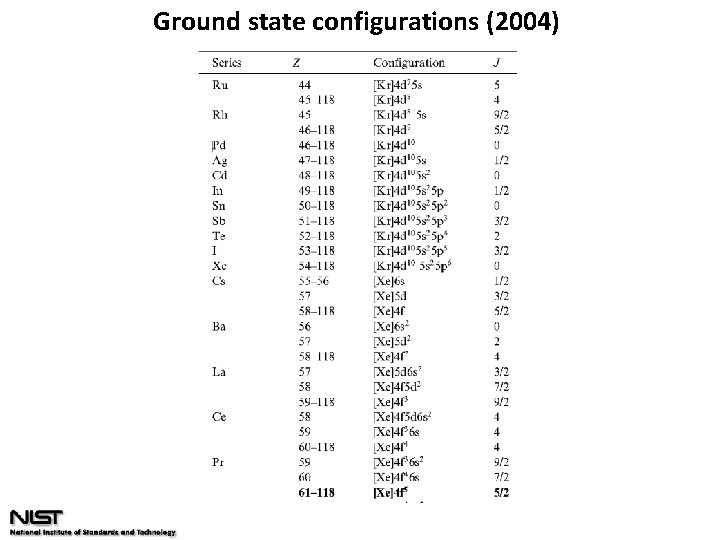
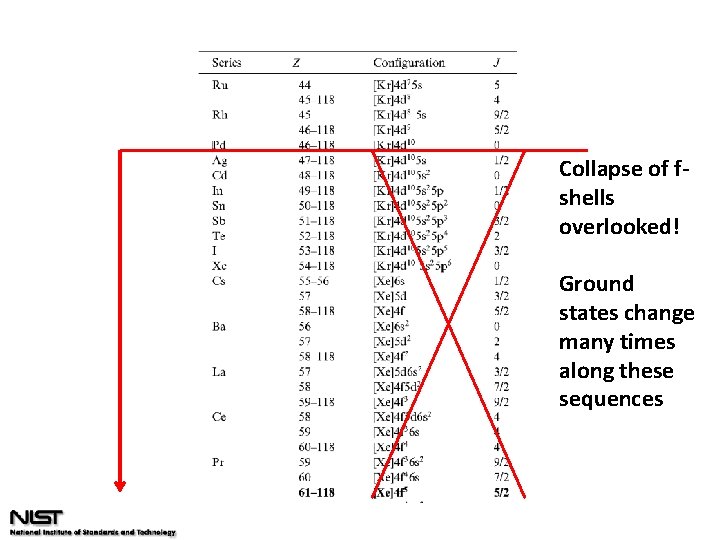
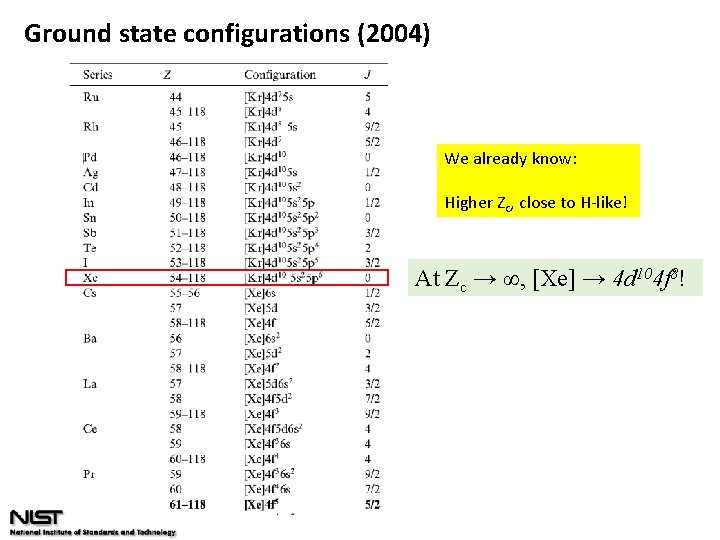
Ground state configurations (2004)

Collapse of fshells overlooked! Ground states change many times along these sequences

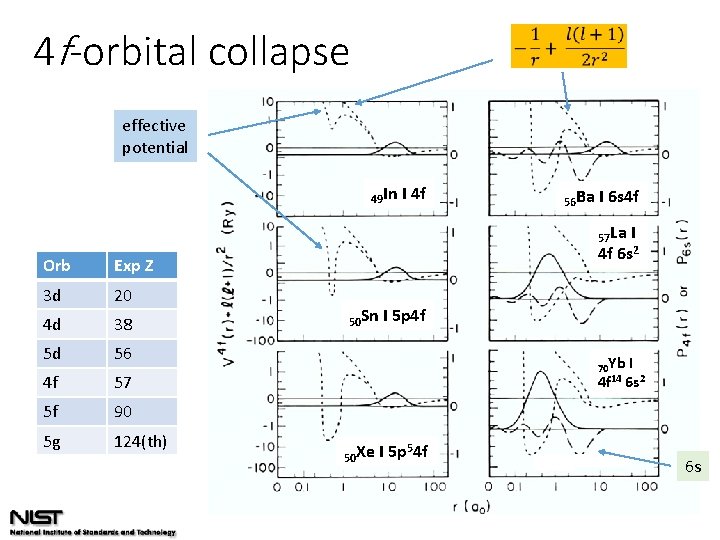
4 f-orbital collapse effective potential 49 In I 4 f 56 Ba I 6 s 4 f 57 La Orb Exp Z 3 d 20 4 d 38 5 d 56 4 f 57 5 f 90 5 g 124(th) I 4 f 6 s 2 50 Sn I 5 p 4 f 70 Yb I 4 f 14 6 s 2 50 Xe I 5 p 54 f 6 s

Ground state configurations (2004) We already know: Higher Zc, close to H-like! At Zc → ∞, [Xe] → 4 d 104 f 8!

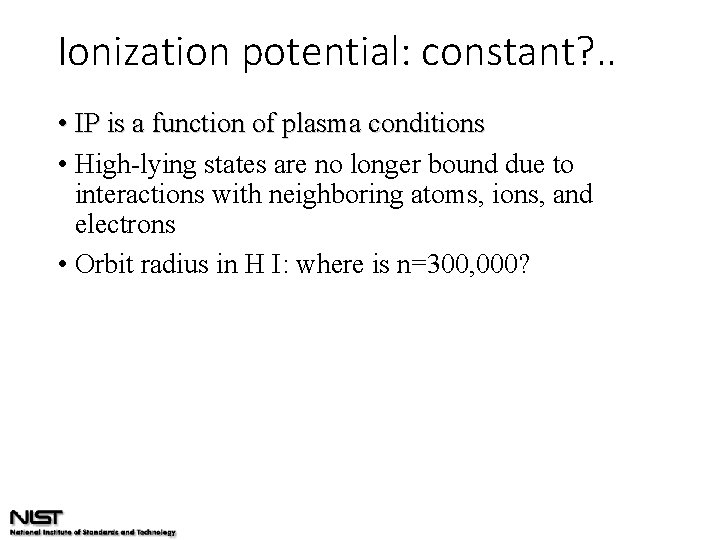
Ionization potential: constant? . . • IP is a function of plasma conditions • High-lying states are no longer bound due to interactions with neighboring atoms, ions, and electrons • Orbit radius in H I: where is n=300, 000?

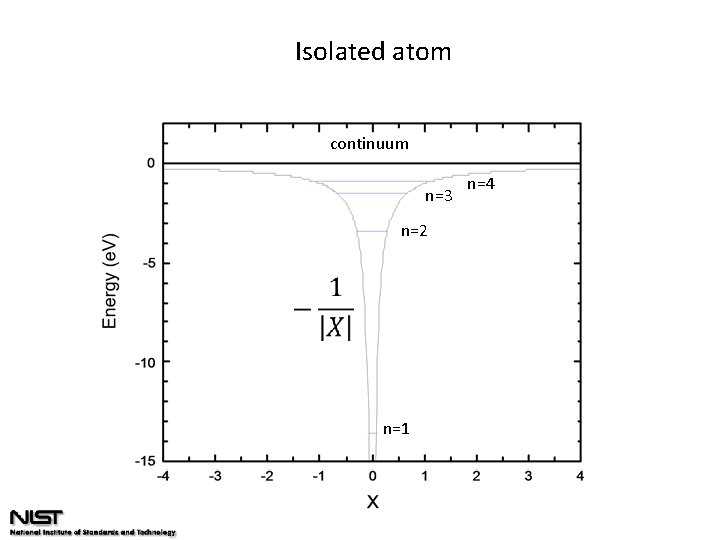
Isolated atom continuum n=3 n=2 n=1 n=4

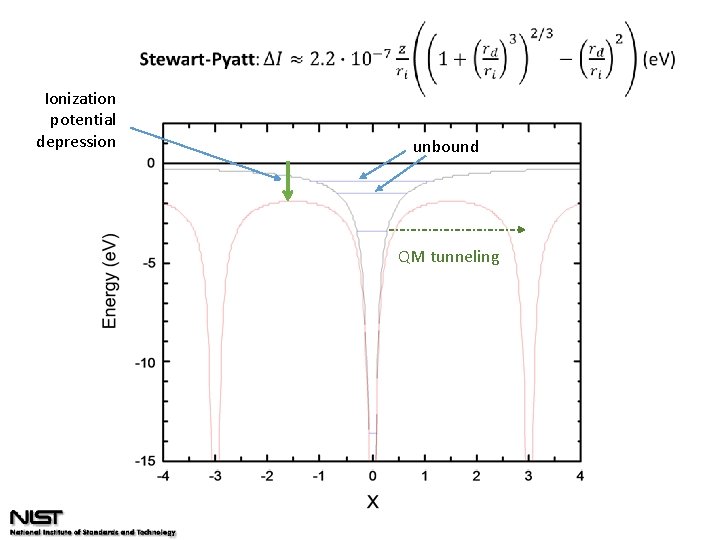
Ionization potential depression unbound QM tunneling

Atomic Structure & Spectra Databases • Extensive list • http: //plasma-gate. weizmann. ac. il/directories/databases/ • Evaluated and recommended data • NIST Atomic Spectra Database http: //physics. nist. gov/asd • Level energies, ionization potentials, spectral lines, transition probabilities • Other data collections • • VALD (Sweden) SPECTR-W 3 (Russia) CAMDB (China) CHIANTI (USA/UK/…) Kurucz databases (USA) GENIE (IAEA) …

ASD • Contents • Procedure: evaluation, analysis • Basic search of energies • Units, ascii, • Term energies • Spectral lines • Multiplets • Grotrian diagrams