Atomic Structure What is an Atom The smallest





- Slides: 13

Atomic Structure

What is an Atom? • The smallest part of an element.

Early models of the atom: Democritus: • Matter is made of tiny indivisible and indestructible particles called atoms. IT’S ALL GREEK TO ME. . . 460 -370 BC

John Dalton - 1803 • Atom is indivisible. • All atoms of a given element are identical. • Conservation of atoms in a chemical reaction.

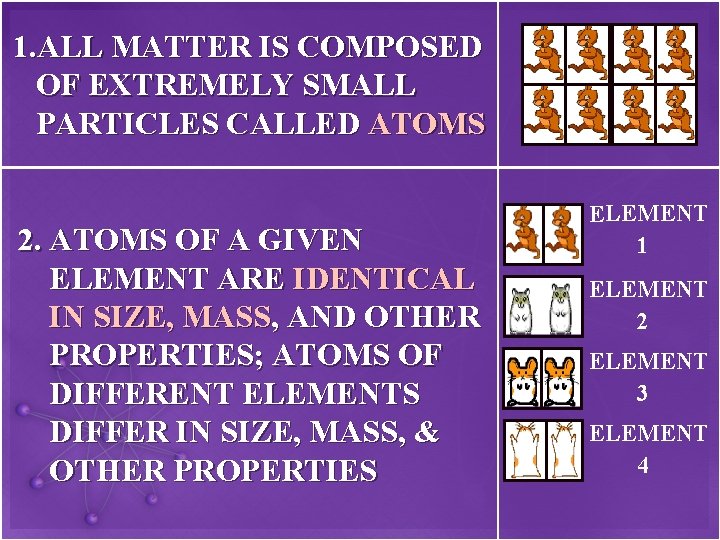
1. ALL MATTER IS COMPOSED OF EXTREMELY SMALL PARTICLES CALLED ATOMS 2. ATOMS OF A GIVEN ELEMENT ARE IDENTICAL IN SIZE, MASS, AND OTHER PROPERTIES; ATOMS OF DIFFERENT ELEMENTS DIFFER IN SIZE, MASS, & OTHER PROPERTIES ELEMENT 1 ELEMENT 2 ELEMENT 3 ELEMENT 4

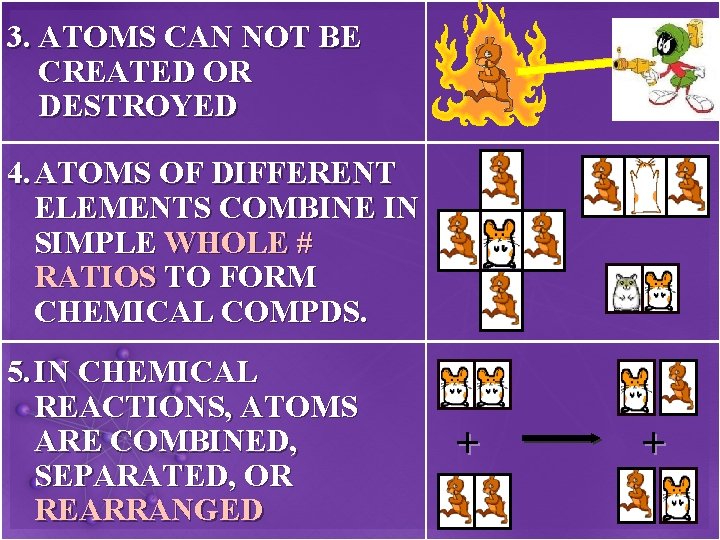
3. ATOMS CAN NOT BE CREATED OR DESTROYED 4. ATOMS OF DIFFERENT ELEMENTS COMBINE IN SIMPLE WHOLE # RATIOS TO FORM CHEMICAL COMPDS. 5. IN CHEMICAL REACTIONS, ATOMS ARE COMBINED, SEPARATED, OR REARRANGED + +

Static Electricity • 1750’s Ben Franklin studied charges. • Two types: Positive and Negative Static Electricity Commercial

• THE FIRST DISCOVERY OF A SUBATOMIC PARTICLE RESULTED FROM THE INVESTIGATIONS INTO THE RELATIONSHIP BETWEEN ELECTRICITY AND MATTER. • IN THE LATE 1800’S, MANY EXPERIMENTS WERE PERFORMED IN WHICH ELECTRIC CURRENT WAS PASSED THROUGH VARIOUS GASES AT LOW PRESSURE. • CARRIED OUT IN TUBES CALLED CATHODE-RAY TUBES

• INVESTIGATORS NOTICED THAT WHEN CURRENT WAS PASSED THROUGH A CATHODE RAY TUBE, THE SURFACE OF THE TUBE DIRECTLY OPPOSITE THE CATHODE GLOWED. Cathode Ray Video

J. J. Thomson 1856 -1940 THOMPSON CONCLUDED THAT ALL CATHODE RAYS ARE I PLAY WITH COMPOSED OF IDENTICAL ELECTRONS NEGATIVELY CHARGED PARTICLES LATER WERE CALLED ELECTRONS.

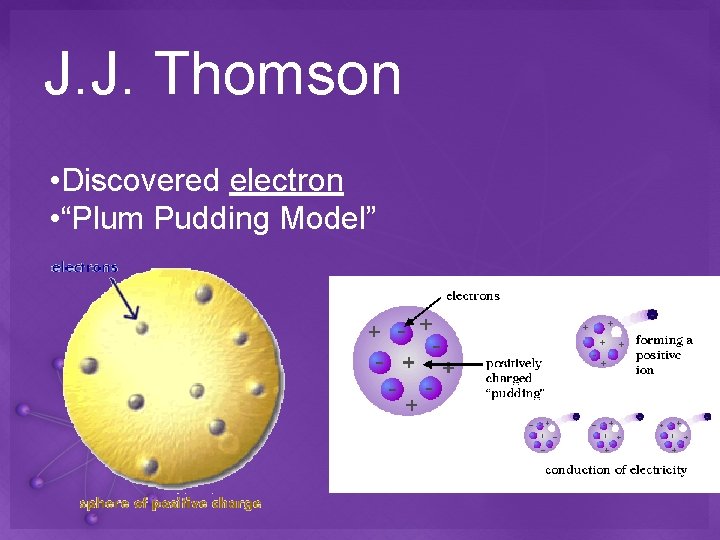
J. J. Thomson • Discovered electron • “Plum Pudding Model”

Ernest Rutherford - 1909 HOW IS THE ATOM BUILT? Gold Foil Experiment

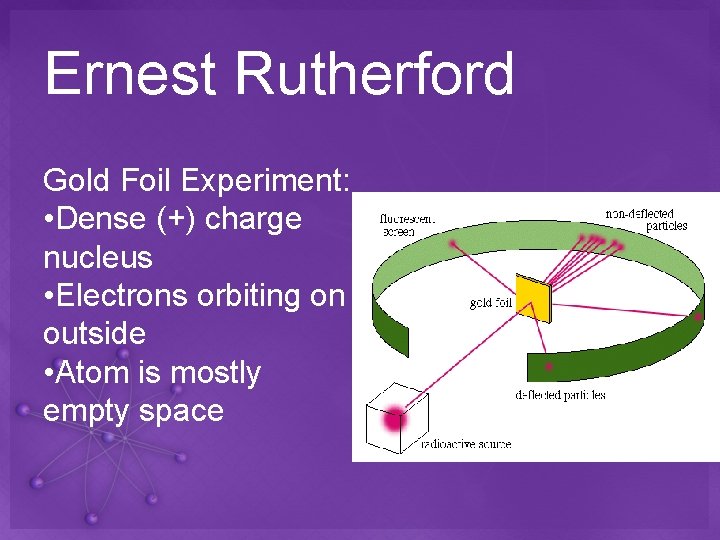
Ernest Rutherford Gold Foil Experiment: • Dense (+) charge nucleus • Electrons orbiting on outside • Atom is mostly empty space