Atomic Structure Unit 4 Ch 4 2 20























- Slides: 49

Atomic Structure Unit 4: Ch 4. 2, 20. 1, 10. 1 -2, 4 Geo Ch 21. 2 -4

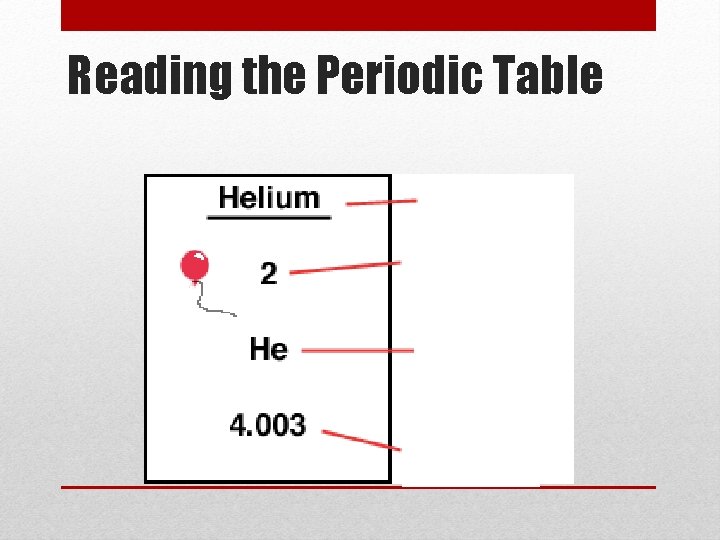
Reading the Periodic Table

Practice - Label this own on your own!

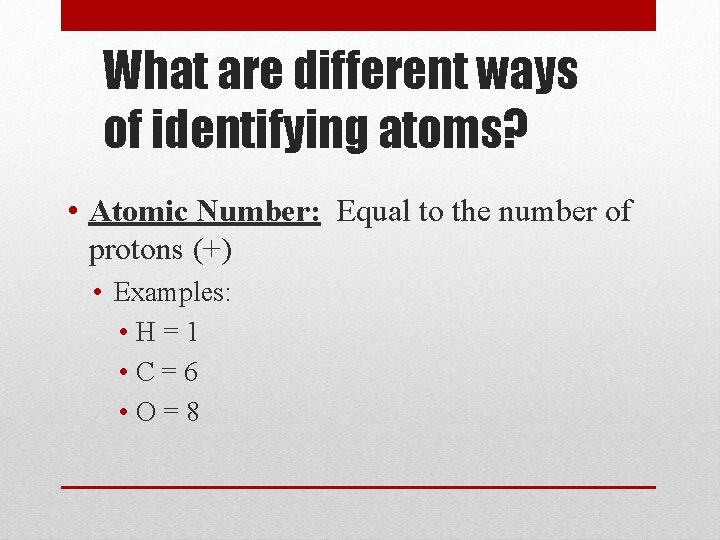
What are different ways of identifying atoms? • Atomic Number: Equal to the number of protons (+) • Examples: • H=1 • C=6 • O=8

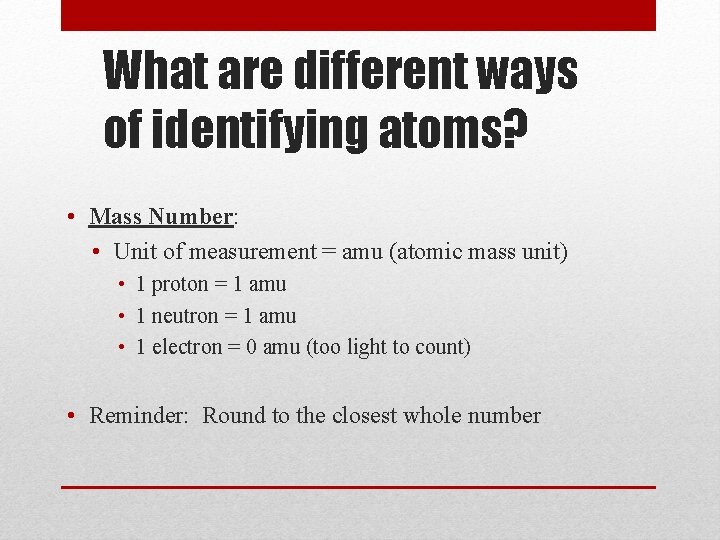
What are different ways of identifying atoms? • Mass Number: • Unit of measurement = amu (atomic mass unit) • 1 proton = 1 amu • 1 neutron = 1 amu • 1 electron = 0 amu (too light to count) • Reminder: Round to the closest whole number

What are different ways of identifying atoms? MASS NUMBER = PROTONS + NEUTRONS or NEUTRONS = MASS NUMBER – ATOMIC NUMBER Example: How many neutrons are in U-236? Neutrons = 236 – 92 = 144 neutrons

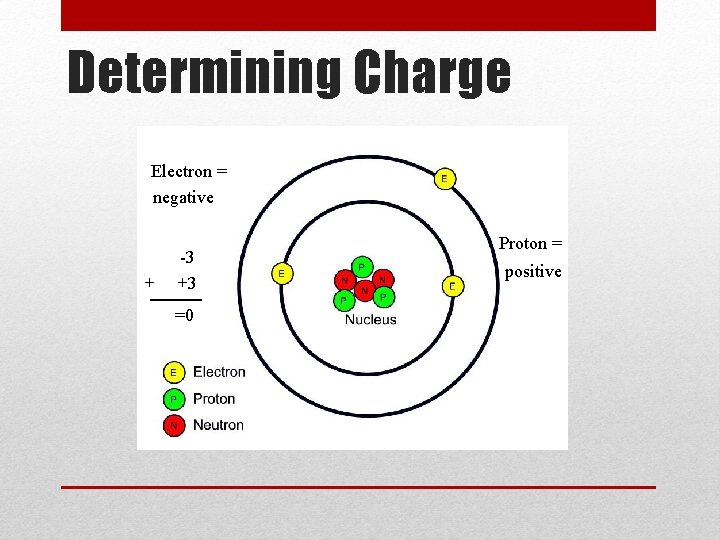
Determining Charge Electron = negative + -3 +3 =0 Proton = positive

Practice – Determine charge on your own!

Ions • The previous example had a charge • An atom that has a charge is called an ion • A positive ion = cation • A negative ion = anion

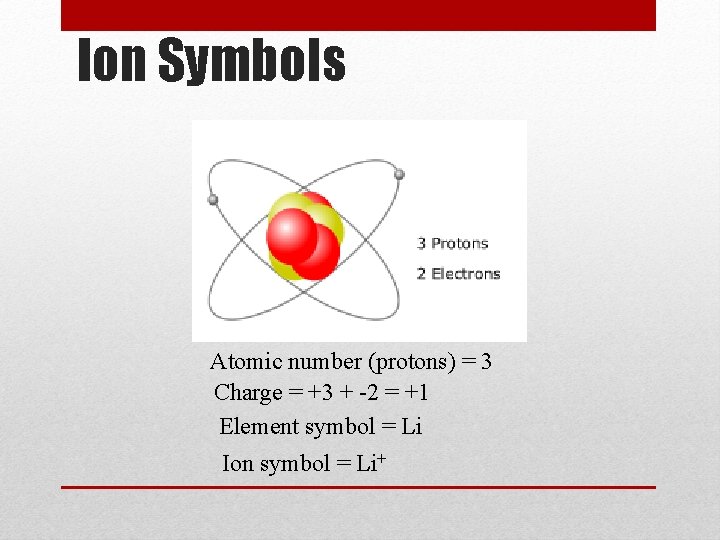
Ion Symbols Atomic number (protons) = 3 Charge = +3 + -2 = +1 Element symbol = Li Ion symbol = Li+

Practice – Write ion symbol on your own!

Making Ions • Changing number of neutrons = changing mass number • Changing number of protons = changing atomic number = different element • Changing number of electrons = making atom charge = ION!

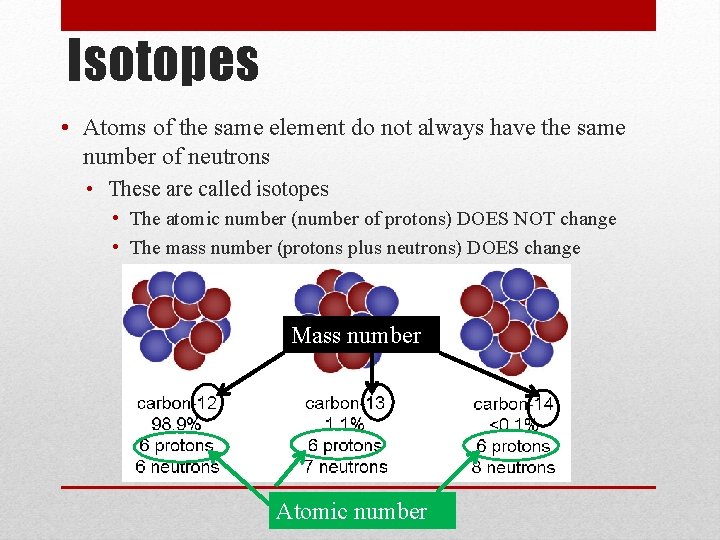
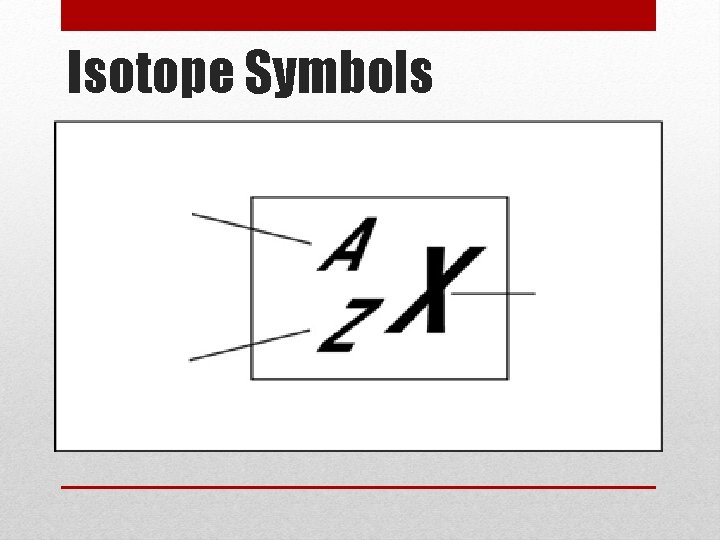
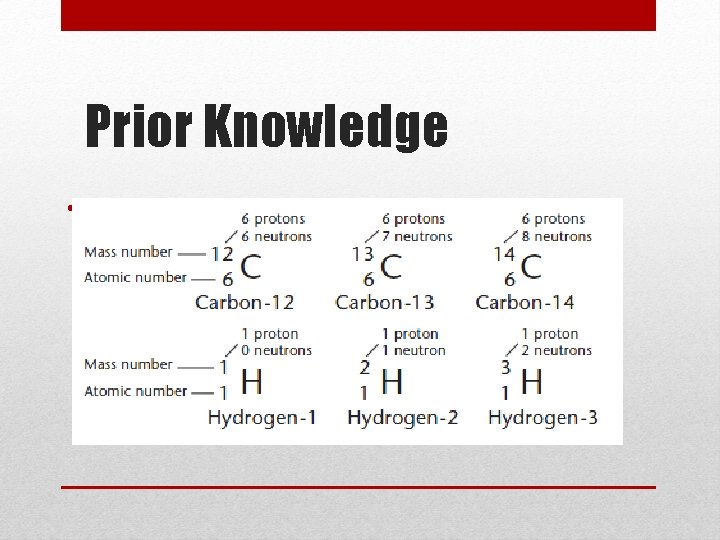
Isotopes • Atoms of the same element do not always have the same number of neutrons • These are called isotopes • The atomic number (number of protons) DOES NOT change • The mass number (protons plus neutrons) DOES change Mass number Atomic number

Isotope Symbols

Isotope Symbols 1. What is the element symbol? 2. What is the name of the element (find on periodic table)? 3. What is the atomic number? 4. What is the mass number? 5. How many protons does this element have? 6. How many neutrons does this element have?

Practice- Answer these questions on your own! 1. What is the element symbol? 2. What is the name of the element (find on periodic table)? 3. What is the atomic number? 4. What is the mass number? 5. How many protons does this element have? 6. How many neutrons does this element have?

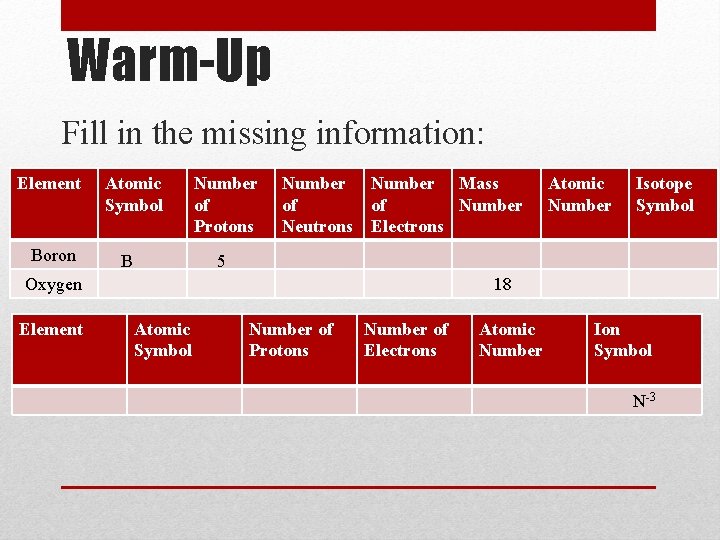
Warm-Up Fill in the missing information: Element Boron Atomic Symbol B Number of Protons Number Mass of of Number Neutrons Electrons Isotope Symbol 5 Oxygen Element Atomic Number 18 Atomic Symbol Number of Protons Number of Electrons Atomic Number Ion Symbol N-3

Electric Charge, • • • Can be positive (protons) Can be negative (electrons) When they are even, the atom is neutral. Some atoms hold their electrons more tightly than others. To become positively charged, atoms must ____ electrons. To become negatively charged, atoms must ____ electrons. Law of Conservation of Charge, force, distance relationship? Conductors vs Insulators Charge by contact vs Charge by induction

Check for Understanding 1. How do you make an object positively charged? 2. How do you make an object negatively charged? 3. What are two ways to charge an object? (Hint: charging by ___ or ___. ) 4. What happens to two positive charges brought near each other (attract/repel)? 5. What happens when a positive and negative are brought near each other (attract/repel)? 6. If two charges are brought closer together, what happens to the force? 7. If one charge is increased, what happens to the force? 8. State the law of conservation of charge.

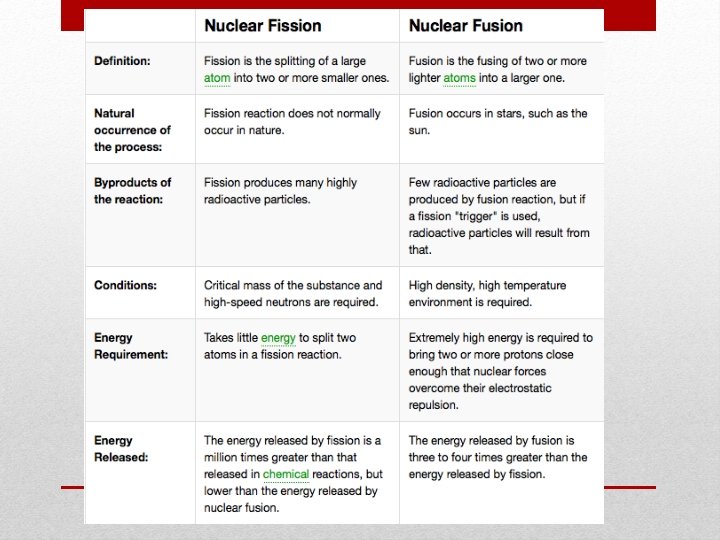
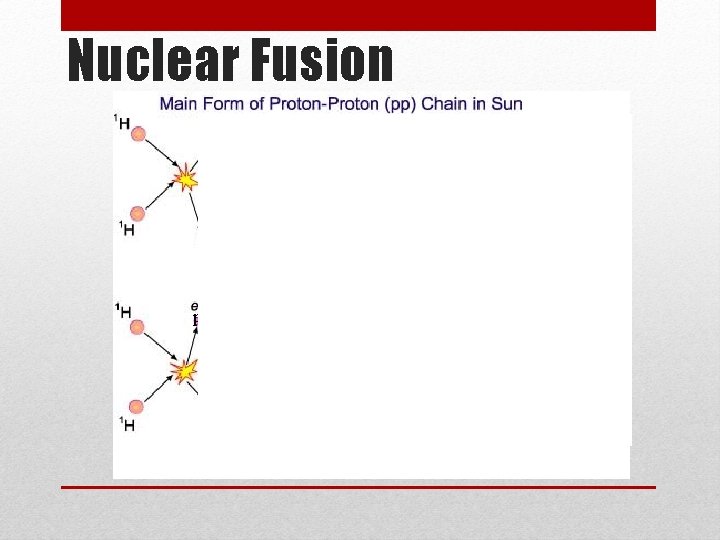
• Nuclear Fission – the process of splitting a nucleus into two nuclei with smaller masses • Chain reaction – ongoing series of fission reactions • Critical mass – the amount of fissionable material required so that each fission reaction produce approximately one more fission reaction • Nuclear Fusion – two nuclei with low masses are combined to form one nucleus of larger mass • Fission and Fusion Video KEY TERMS: Ch 10. 4 Nuclear Reactions


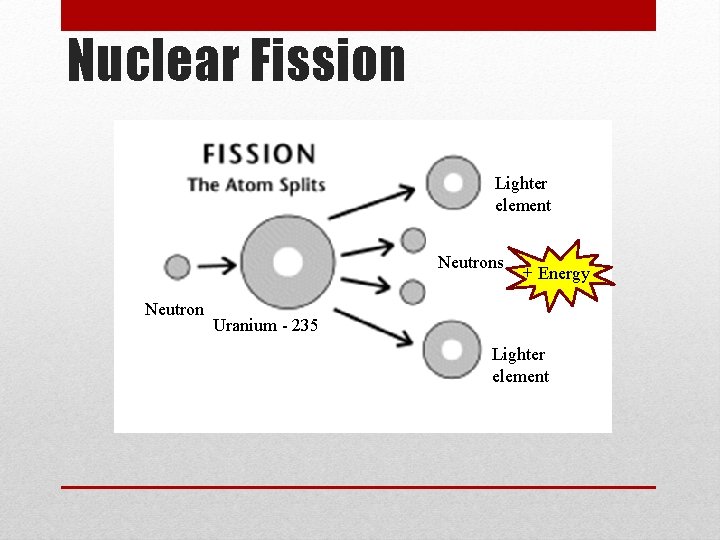
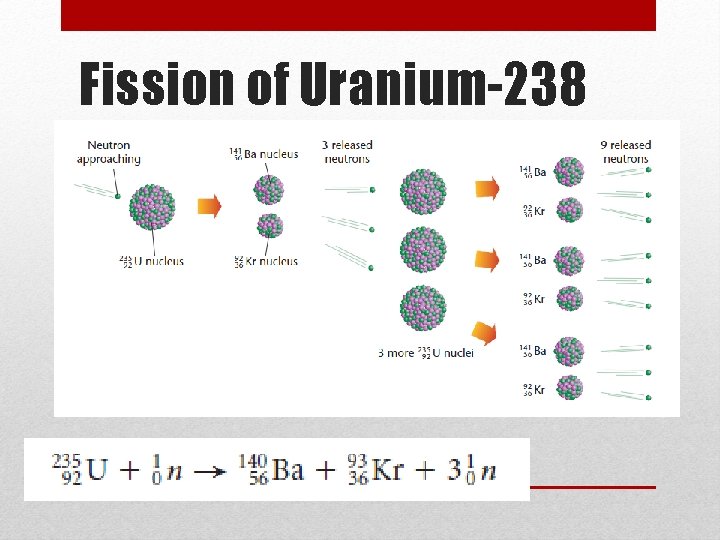
Nuclear Fission • Fission means “to divide” • The process of splitting a nucleus into two nuclei with smaller masses • This occurs when a neutron hits the nucleus of an atom • Only large nuclei can undergo fission reactions • Two uses from book: generate electricity, nuclear weapons • The total mass of the products is slightly less than the mass of the original nuclear and the neutrons that break free • The missing mass is converted into large amounts of energy

Nuclear Fission Lighter element Neutrons Neutron + Energy Uranium - 235 Lighter element

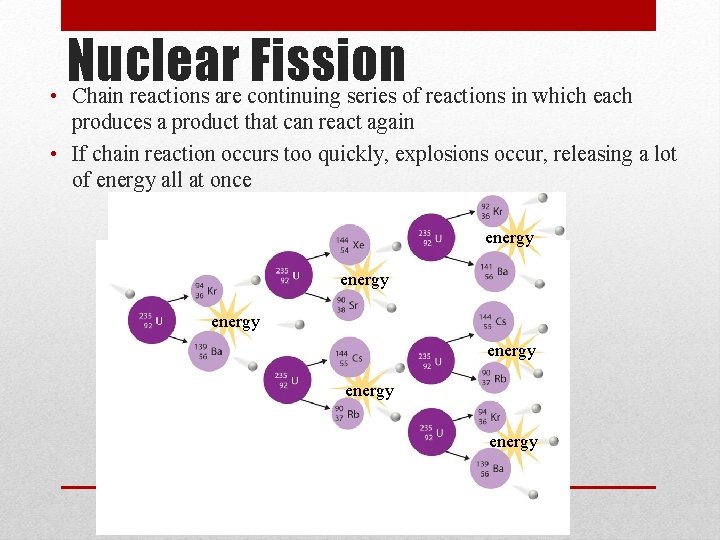
Nuclear Fission • Chain reactions are continuing series of reactions in which each produces a product that can react again • If chain reaction occurs too quickly, explosions occur, releasing a lot of energy all at once energy energy

Fission of Uranium-238

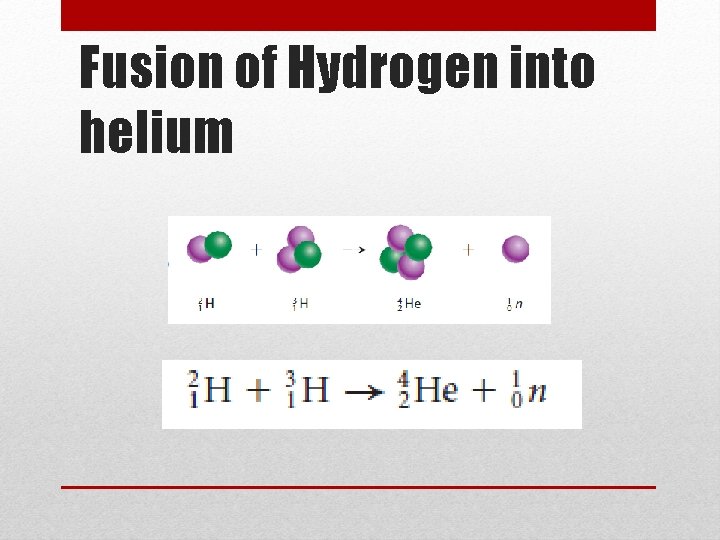
Nuclear Fusion • Fusion = combine • Nuclear Fusion is when two or more nuclei combine to form a larger nucleus (requires very high temps to overcome the repulsive forces) • The sun uses this process to produce energy • It fuses hydrogen into helium • The problem lies in the energy involved to start fusion • Most nuclei that can undergo fusion are fairly unreactive • Energy given off (output) does not outweigh the energy needed (input) • Hydrogen Bomb • Just like in fission, a small amount of mass is converted into a large amount of energy

Fusion of Hydrogen into helium

Nuclear Fusion positron energy neutrino

Energy to Mass Conversions • Think back to the law of conservation of energy • We need to include mass in this law when talking about fission and fusions reactions • This relationship is shown by Einstein’s theory of relativity • This states that energy and mass are equivalent and can be converted using the equation E=mc 2

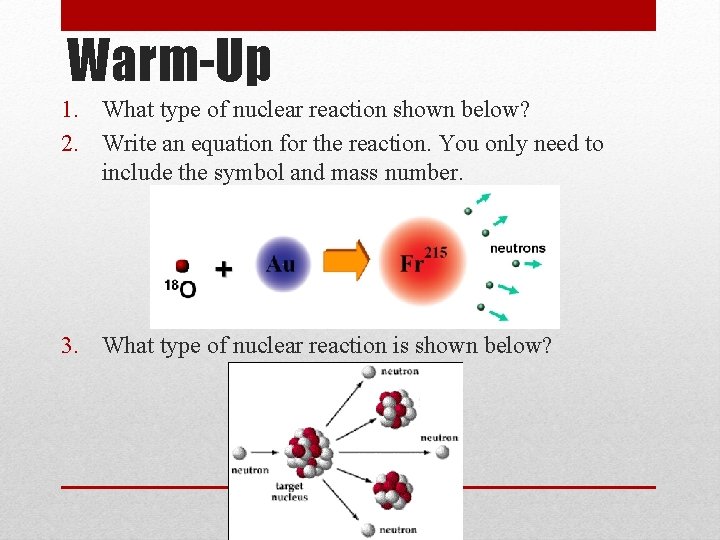
Warm-Up 1. What type of nuclear reaction shown below? 2. Write an equation for the reaction. You only need to include the symbol and mass number. 3. What type of nuclear reaction is shown below?

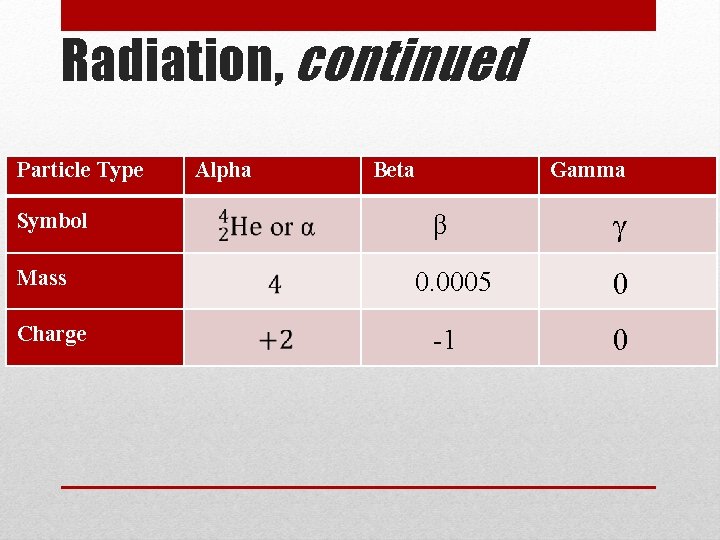
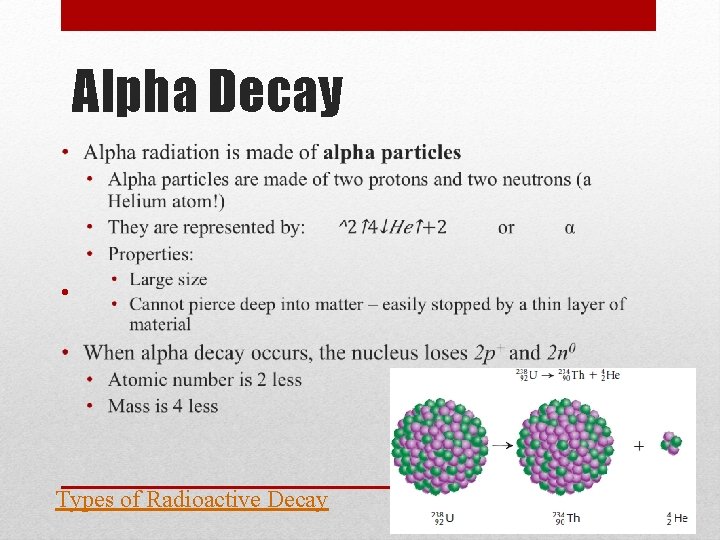
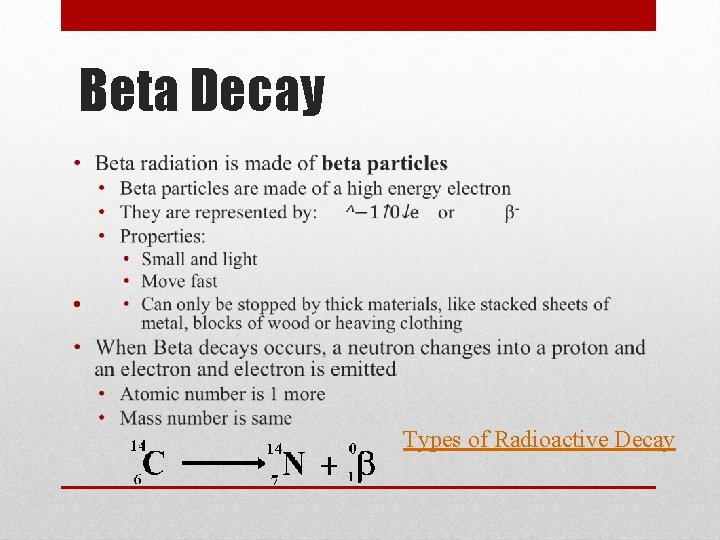
Radiation Physics - Radioactivity: The Discovery of Radioactivity • When a nucleus becomes unstable, it decays releasing particles and energy called nuclear radiation • Radiation comes from radioactive isotopes, or radioisotopes • The release of radiation is called decay • Some radiation is more powerful than others • There are three types of nuclear radiation – • alpha (α) – made of 2 p+ and 2 n 0 • beta (β) – n 0 decays into p+ and e- (beta particle) • gamma (γ) – no mass or charge, just energy

Radiation, continued • Alpha particles • Large • Cannot pierce deep into matter – easily stopped by thin layer of material • Beta Particles • beta particle (small, light, fast) • Can only be stopped by thick materials, like stacked sheets of metal, blocks of wood or heaving clothing • Gamma Radiation • Travels in waves as electromagnetic radiation (or light) • Can pass through most types of materials – need a material thicker than blocks of concrete Types of Radioactive Decay

Radiation, continued Particle Type Symbol Mass Charge Alpha Beta Gamma β 0. 0005 -1 γ 0 0

Prior Knowledge • Isotope Notation

Alpha Decay • Types of Radioactive Decay

Beta Decay • Types of Radioactive Decay

Gamma Decay • Gamma radiation is made of gamma rays • Gamma rays are a high-energy form of electromagnetic radiation • They are represented by: γ • Properties: • No mass or charge • Can pass through most types of materials – need a material thicker than blocks of concrete • When gamma radiation occurs, only energy is given off • Usually occurs along with another type of decay • Atomic number is same • Mass number is same Types of Radioactive Decay

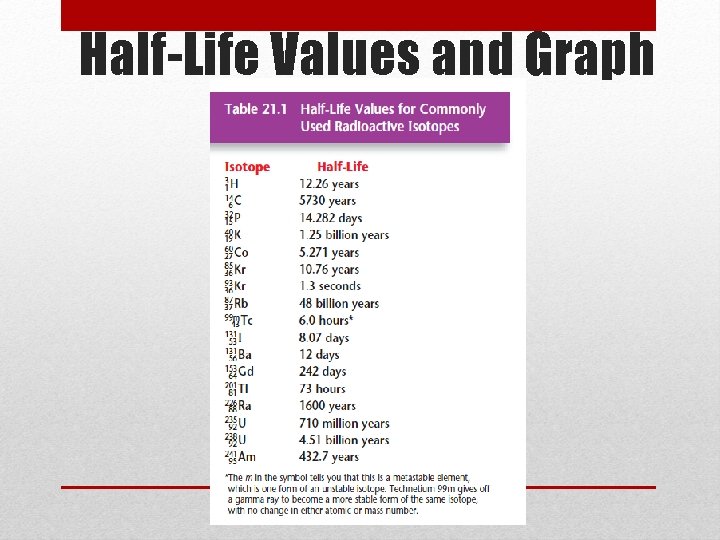
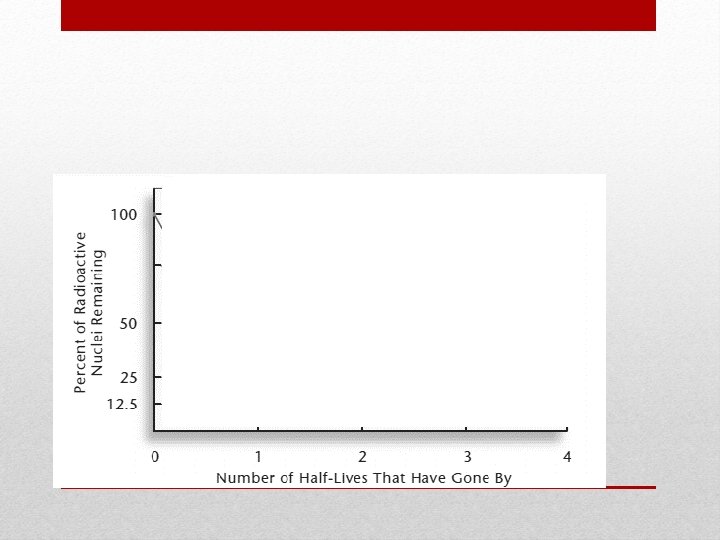
Radiation, continued • Half-life • The amount of time it takes for half of the nuclei of a radioactive isotope to decay • The original substance is called the parent • The new substance is called the daughter

Half-Life Notes •

Half-Life Values and Graph

½ or 1: 1 ratio ¼ or 1: 3 ratio 1/8 or 1: 7 ratio Half-Life Values and Graph

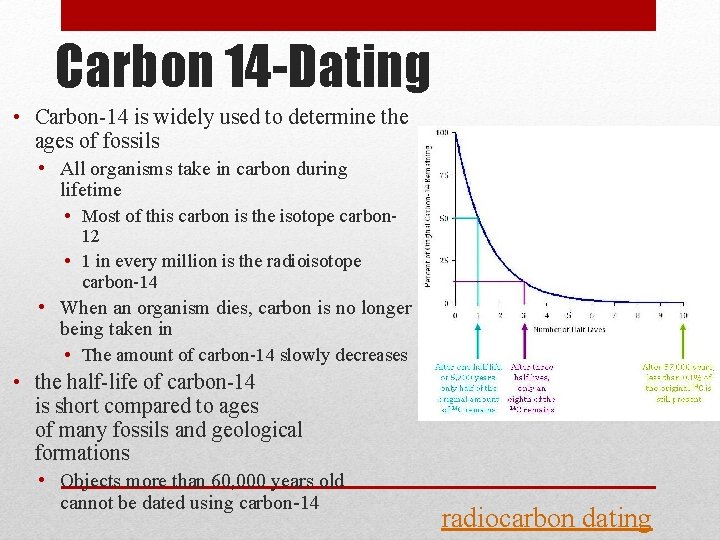
Carbon 14 -Dating • Carbon-14 is widely used to determine the ages of fossils • All organisms take in carbon during lifetime • Most of this carbon is the isotope carbon 12 • 1 in every million is the radioisotope carbon-14 • When an organism dies, carbon is no longer being taken in • The amount of carbon-14 slowly decreases • the half-life of carbon-14 is short compared to ages of many fossils and geological formations • Objects more than 60, 000 years old cannot be dated using carbon-14 radiocarbon dating

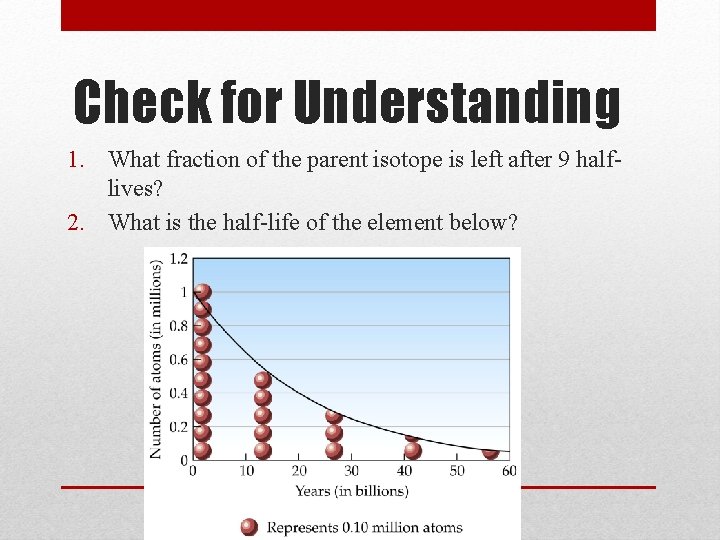
Check for Understanding 1. What fraction of the parent isotope is left after 9 halflives? 2. What is the half-life of the element below?

Practice Example Problem: Ac has a half life of 6. 13 hours. How much of a 5. 0 mg sample would remain after one day (24 hours)? 228 1. The first step is to determine the number of half lives that have elapsed. number of half lives = total time / half-life = 24 hours/6. 13 hours = 3. 9 half lives = 4 (rounded) 2. For each half life, the total amount of the isotope is reduced by half. Amount remaining = Original amount x half-life fraction = 5. 0 mg x 1/16 = 0. 3 mg

Practice 1. Americium-242 has a half-life of 6 hours. If you started with 24 g and you now have 1. 5 g, how much time has passed? 1. 5 g ÷ 24 g = 1/16 left = 4 half lives 4 x 6 hrs = 24 hrs 2. The half-life of cobalt-60 is 5 years. If you have 10 grams of Co-60, how much do you have after 15 years? 15 years ÷ 5 years = 3 half lives 10 g 5 g 2. 5 g 1. 25 g 1 st ½ 2 nd ½ 3 rd ½

CFU Answer these questions on the ¼ piece of paper 1. Gold-198 has a half-life of 2. 7 days. How much of a 96 g sample of gold-198 will be left after 8. 1 days? 2. What is the half-life of a 100. 0 g sample of nitrogen-16 that decays to 12. 5 g of nitrogen-16 in 20. 0 s? (Hint: 12. 5 is 12. 5% of 100. 0, so figure out what fraction that is!) 3. The half-life of Zn-71 is 2. 4 minutes. If one had 100. 0 g at the beginning, how many grams would be left after 7. 2 minutes has elapsed?

Review: Types of Radioactive Decay and Nuclear Equations Videos: • Types of Radioactive Decay and their effects on the Nucleus • Balancing Nuclear Equations and Predicting Products of Nuclear Reactions

Warm-Up 1. What is the electron that is produced when a neutron decays called? (choose from: alpha particle/beta particle/gamma radiation) 2. What is a helium nucleus with two protons and two neutrons called? (choose from: alpha particle/beta particle/gamma radiation) 3. How many half-lives have passed when 1/16 of the parent material remains? 4. What happens to the atomic number and mass number when a beta particle is emitted? 5. What happens to the atomic number and mass number when an alpha particle is emitted?

Warm-Up 1. 2. 3. 4. What is fusion? What is fission? What is radiation/radioactivity? Where does the energy produced in fission and fusion reactions come from?