Atomic Structure To study hydrogen atom To study


- Slides: 12

Atomic Structure • To study “hydrogen atom” • To study how an external field can “split” degenerate ml states—the Zeeman Effect • To study electron spin • To consider adjustments and changes that occur with many-electron atoms

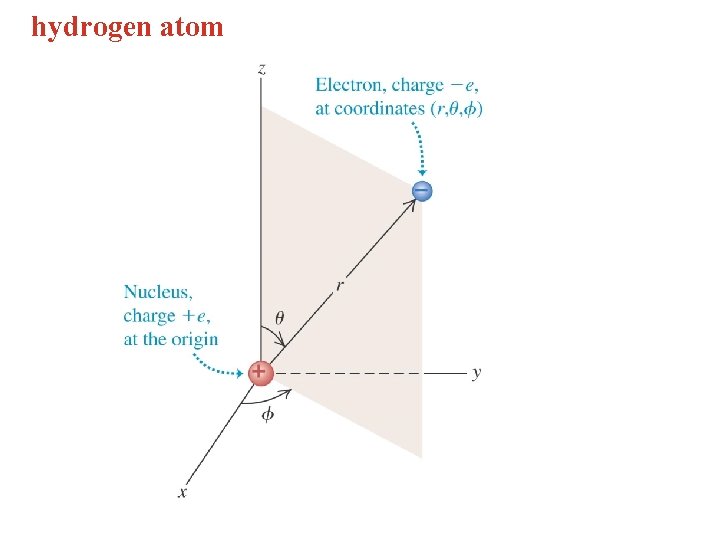
hydrogen atom

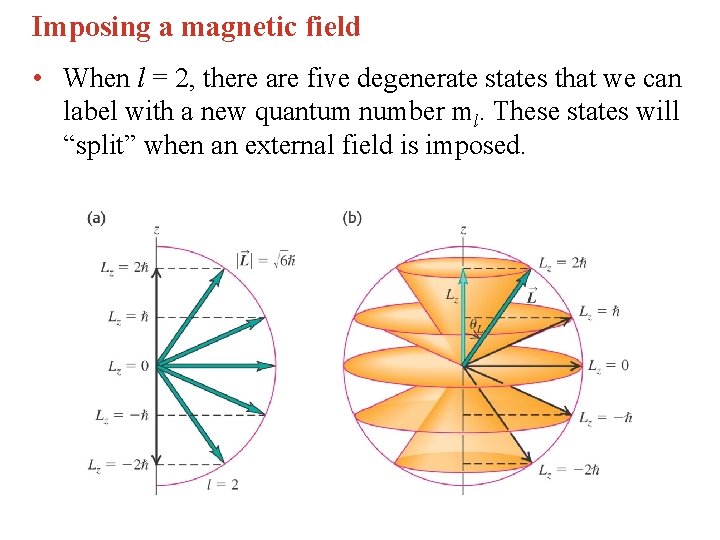
Imposing a magnetic field • When l = 2, there are five degenerate states that we can label with a new quantum number ml. These states will “split” when an external field is imposed.

Details of electron organization • Gathering what we know so far, we have quantum n, l and ml. Below table summarizes this information.

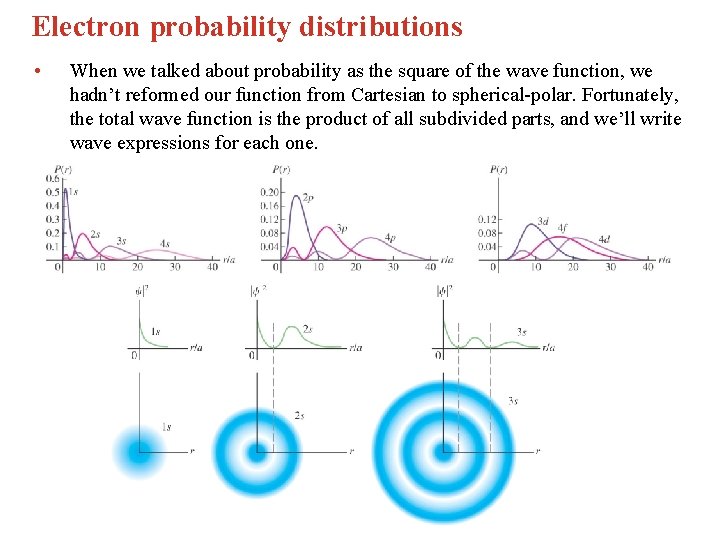
Electron probability distributions • When we talked about probability as the square of the wave function, we hadn’t reformed our function from Cartesian to spherical-polar. Fortunately, the total wave function is the product of all subdivided parts, and we’ll write wave expressions for each one.

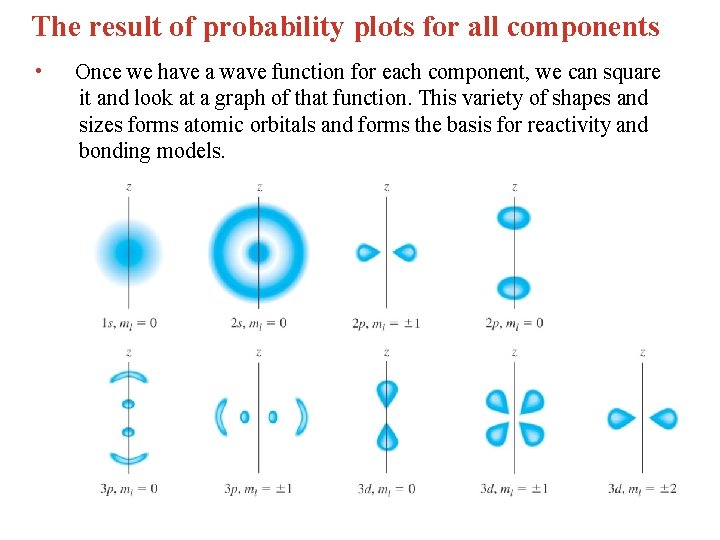
The result of probability plots for all components • Once we have a wave function for each component, we can square it and look at a graph of that function. This variety of shapes and sizes forms atomic orbitals and forms the basis for reactivity and bonding models.

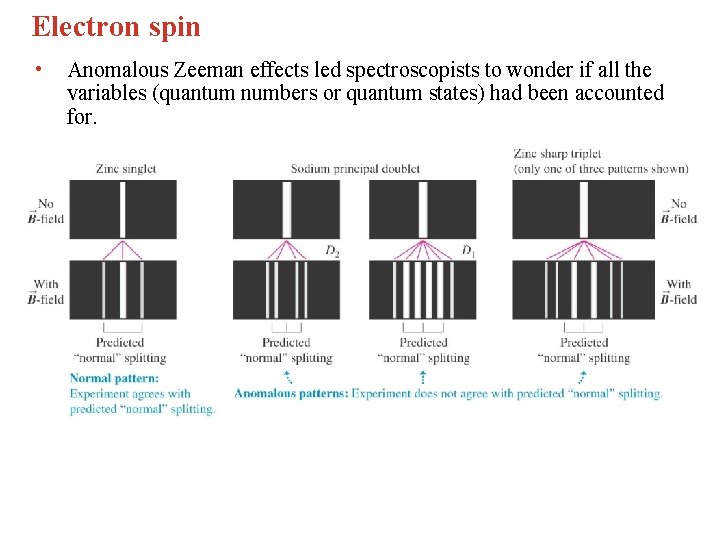
Electron spin • Anomalous Zeeman effects led spectroscopists to wonder if all the variables (quantum numbers or quantum states) had been accounted for.

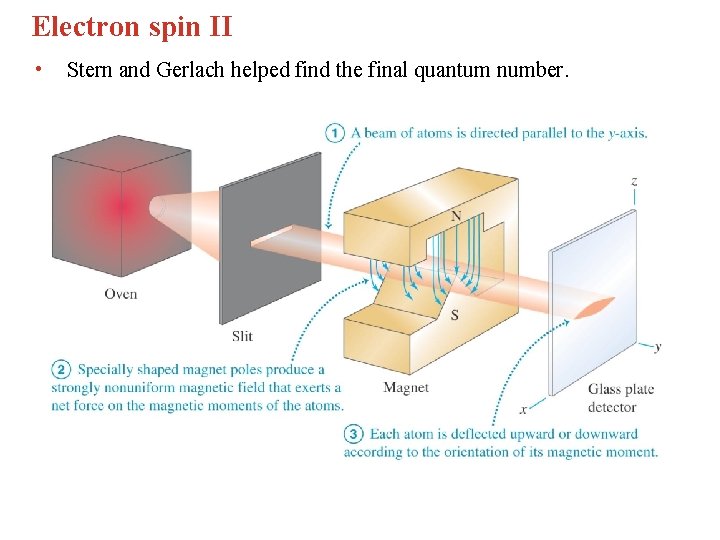
Electron spin II • Stern and Gerlach helped find the final quantum number.

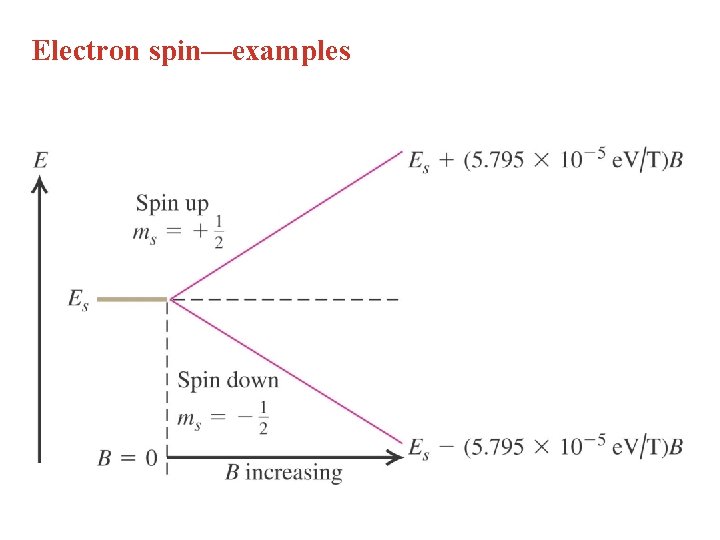
Electron spin—examples

How would it look for an entire atom? • Follow Figures below for a “complete sketch” of the electrons resident in a lithium atom.

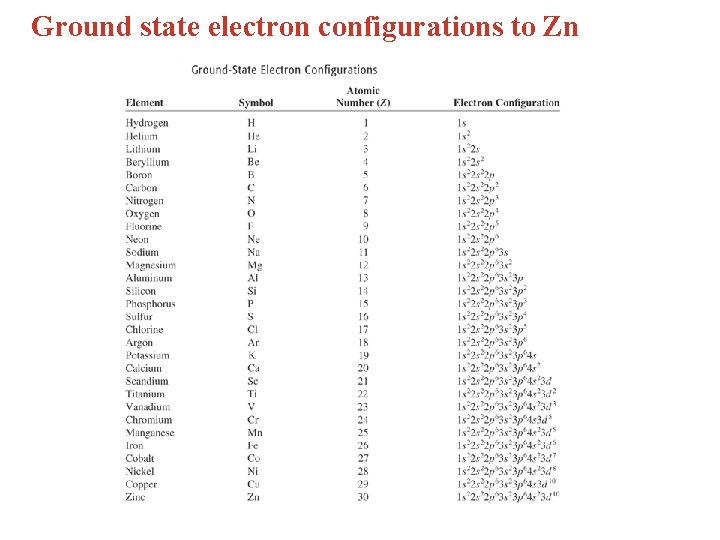
Ground state electron configurations to Zn

Vocabulary: Atomic Structure=atomik yapı Location=Yer, konum External field=dış alan Spin up= spin yukarı Split=bölünme Spin down=spin aşağı Impose=yükleme Orientation=yönlendirme State=durum Beam=ışın Shell=kabuk Subshell=altkabuk Electron probability distributions=Elektron olasılık dağılımları Bonding models=bağ modelleri Zinc=Çinko Anomalous=anormal Pattern=desen Oven=fırın Deflect=sapmak