Atomic Structure The structure of the atom The



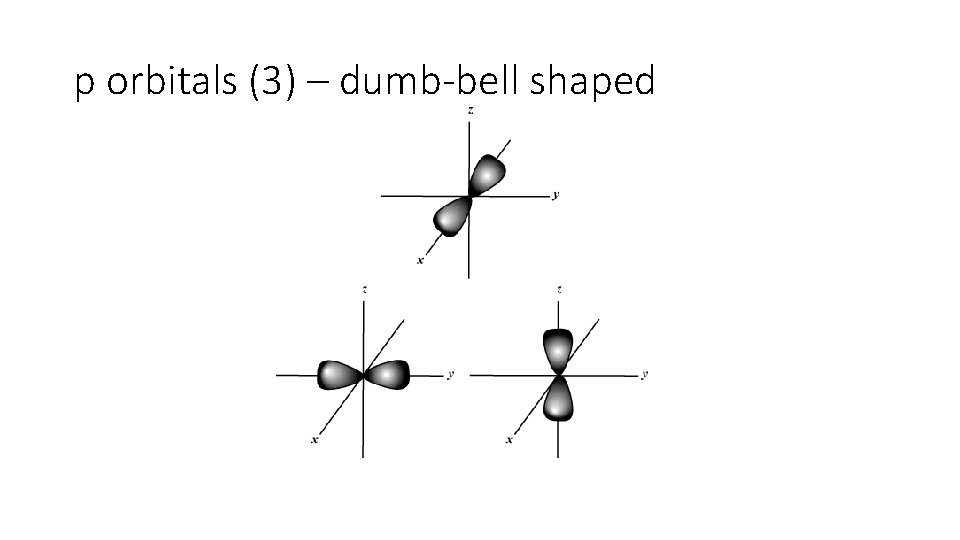


- Slides: 27

Atomic Structure

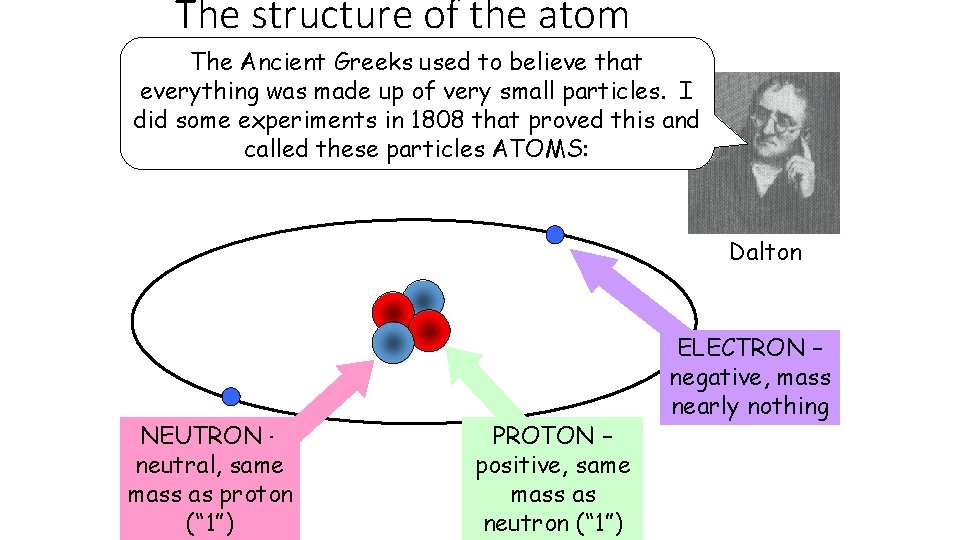
The structure of the atom The Ancient Greeks used to believe that everything was made up of very small particles. I did some experiments in 1808 that proved this and called these particles ATOMS: Dalton NEUTRON – neutral, same mass as proton (“ 1”) PROTON – positive, same mass as neutron (“ 1”) ELECTRON – negative, mass nearly nothing

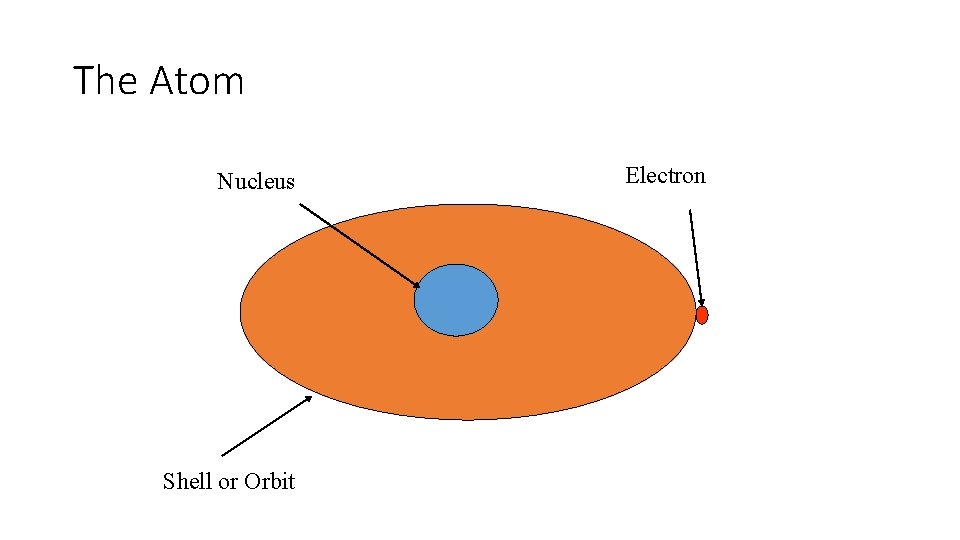
The Atom Nucleus Shell or Orbit Electron

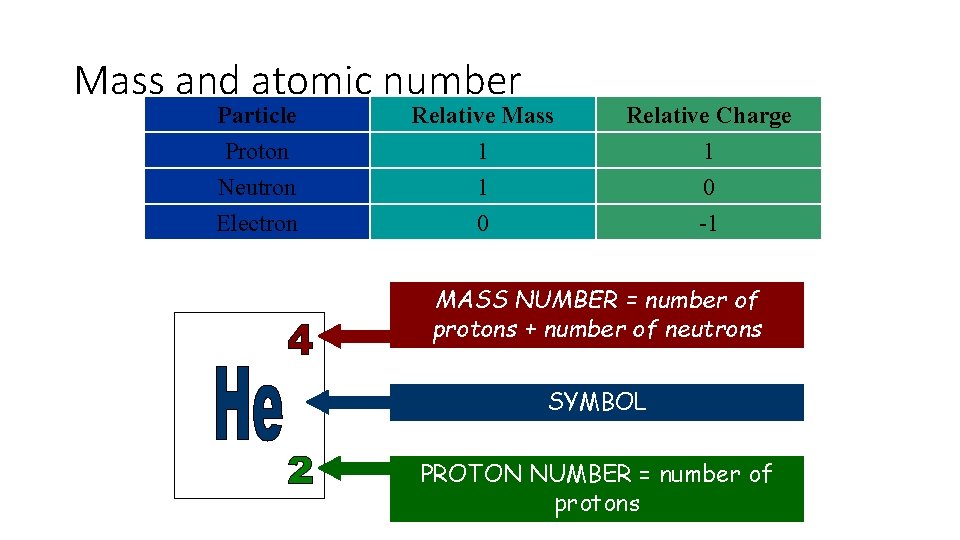
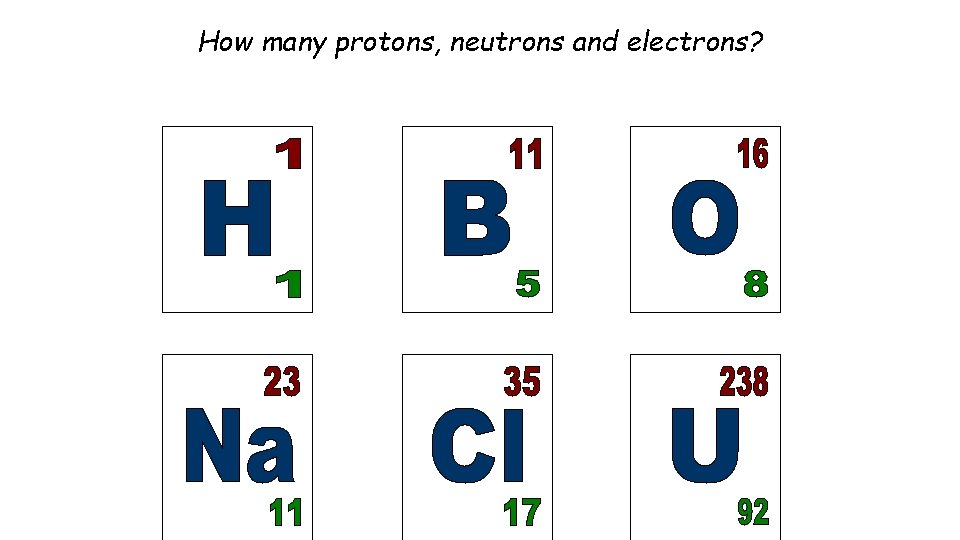
Mass and atomic number Particle Proton Neutron Electron Relative Mass 1 1 0 Relative Charge 1 0 -1 MASS NUMBER = number of protons + number of neutrons SYMBOL PROTON NUMBER = number of protons

How many protons, neutrons and electrons?

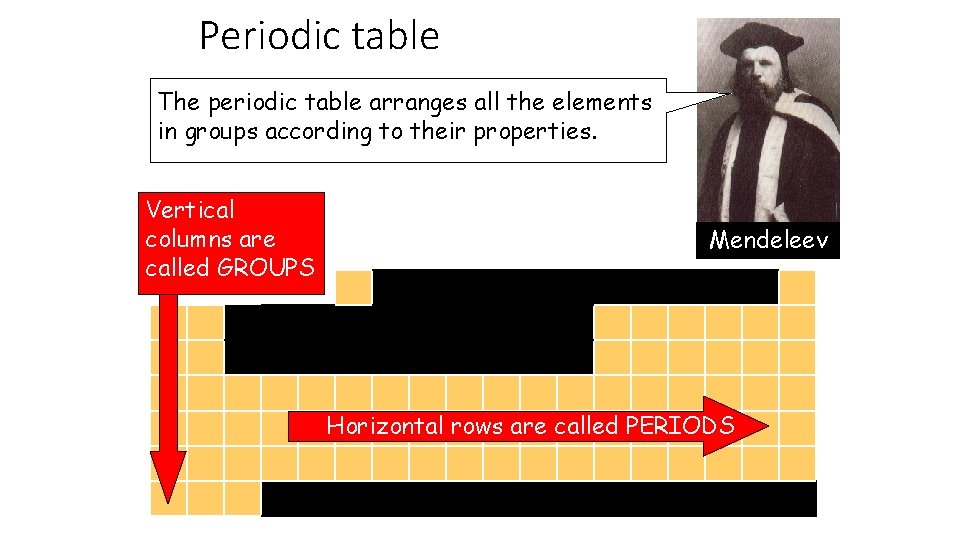
Periodic table The periodic table arranges all the elements in groups according to their properties. Vertical columns are called GROUPS Mendeleev Horizontal rows are called PERIODS

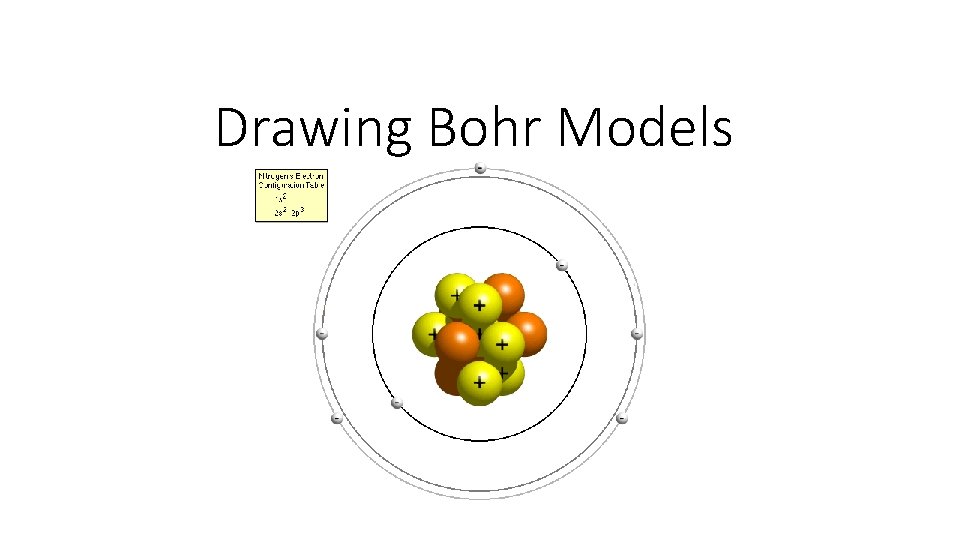
Drawing Bohr Models

Bohr Models 1. Bohr models are used to predict reactivity in elements. 2. Reactivity refers to how likely an element is to form a compound with another element. 3. When looking at Bohr models, we look at its valence electrons (the electrons on the last energy level) to determine reactivity.

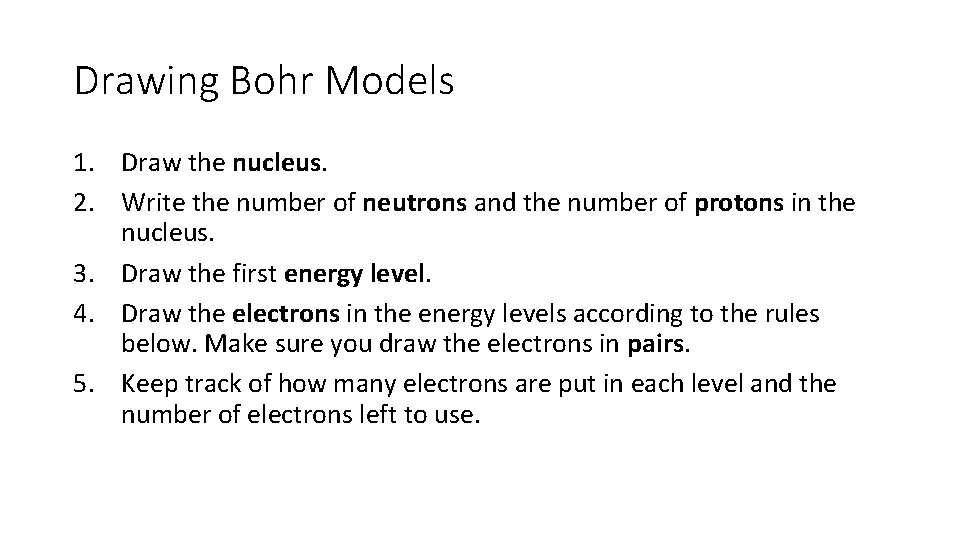
Drawing Bohr Models 1. Draw the nucleus. 2. Write the number of neutrons and the number of protons in the nucleus. 3. Draw the first energy level. 4. Draw the electrons in the energy levels according to the rules below. Make sure you draw the electrons in pairs. 5. Keep track of how many electrons are put in each level and the number of electrons left to use.

Rules for Energy Levels 1. 2. 3. 4. Level 1 (closest to the nucleus) can hold a maximum of 2 e. Level 2 can hold a max of 8 e. Level 3 can hold a max of 18 e. Level 4 can hold a max of 32 e. You must fill one level before going on to draw the next level!

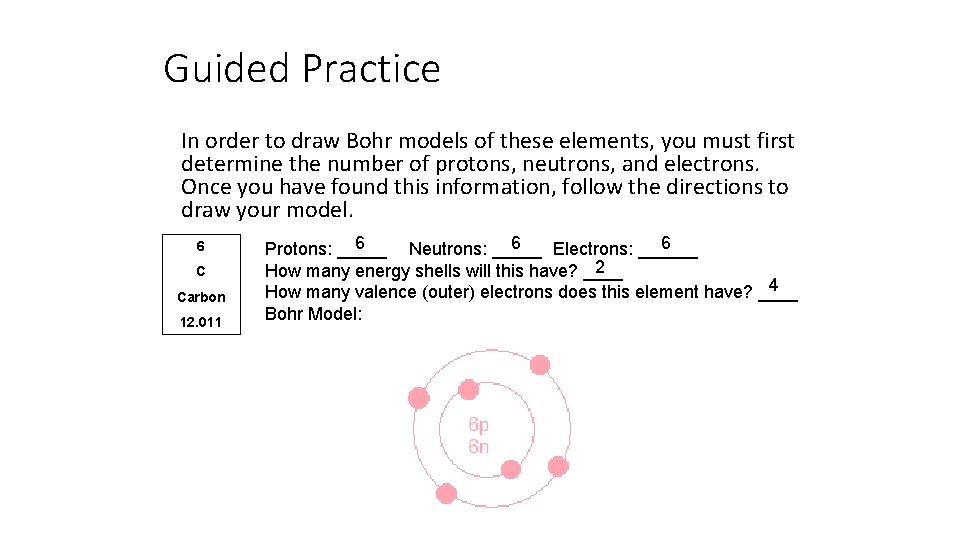
Guided Practice In order to draw Bohr models of these elements, you must first determine the number of protons, neutrons, and electrons. Once you have found this information, follow the directions to draw your model. 6 C Carbon 12. 011 6 6 6 Protons: _____ Neutrons: _____ Electrons: ______ 2 How many energy shells will this have? ____ 4 How many valence (outer) electrons does this element have? ____ Bohr Model:

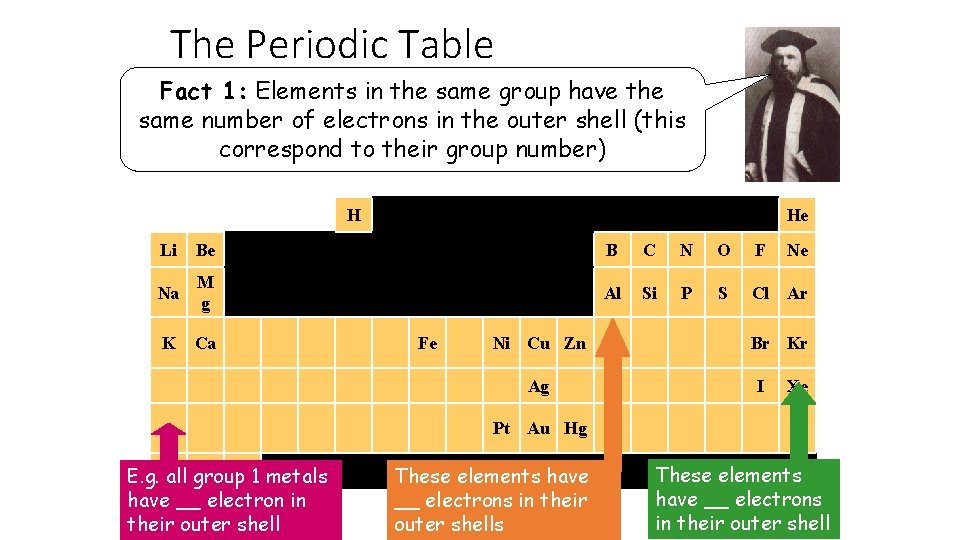
The Periodic Table Fact 1: Elements in the same group have the same number of electrons in the outer shell (this correspond to their group number) H He Li Be B C N O F Na M g Al Si P S Cl Ar K Ca Fe Ni Cu Zn Ag Ne Br Kr I Xe Pt Au Hg E. g. all group 1 metals have __ electron in their outer shell These elements have __ electrons in their outer shells These elements have __ electrons in their outer shell

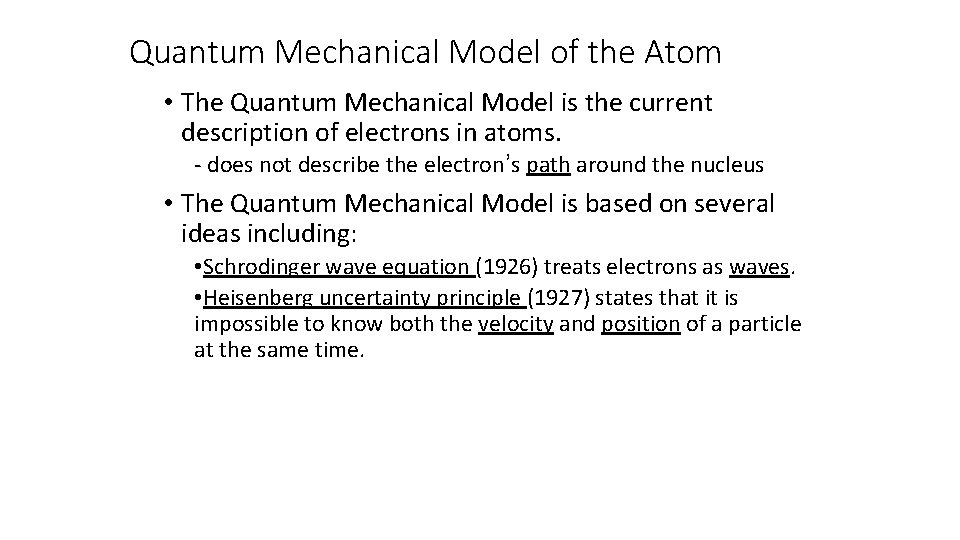
Quantum Mechanical Model of the Atom • The Quantum Mechanical Model is the current description of electrons in atoms. - does not describe the electron’s path around the nucleus • The Quantum Mechanical Model is based on several ideas including: • Schrodinger wave equation (1926) treats electrons as waves. • Heisenberg uncertainty principle (1927) states that it is impossible to know both the velocity and position of a particle at the same time.


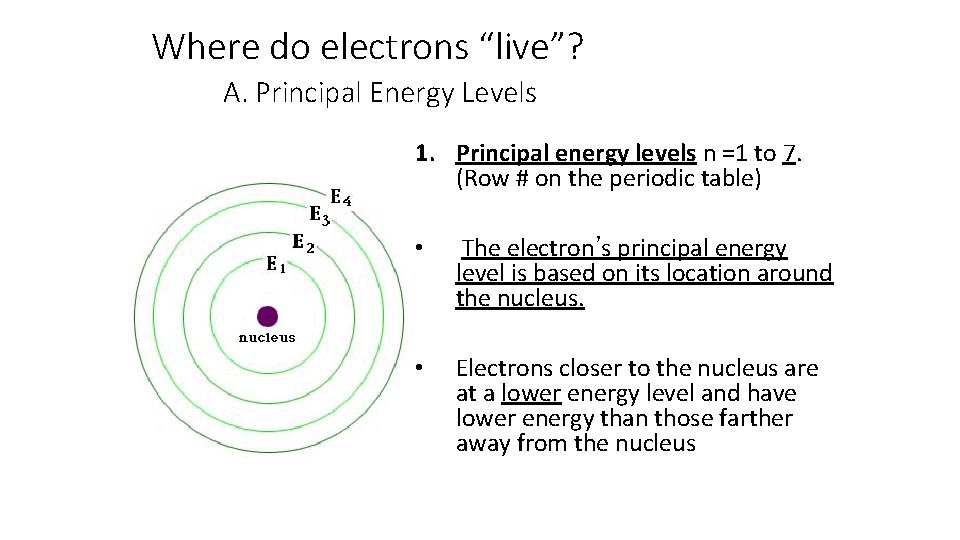
Where do electrons “live”? A. Principal Energy Levels 1. Principal energy levels n =1 to 7. (Row # on the periodic table) • The electron’s principal energy level is based on its location around the nucleus. • Electrons closer to the nucleus are at a lower energy level and have lower energy than those farther away from the nucleus

Energy levels are like rungs of a ladder. You cannot be in between a rung Energy levels in an atom’s electron are unequally spaced. The higher energy levels are closer together.

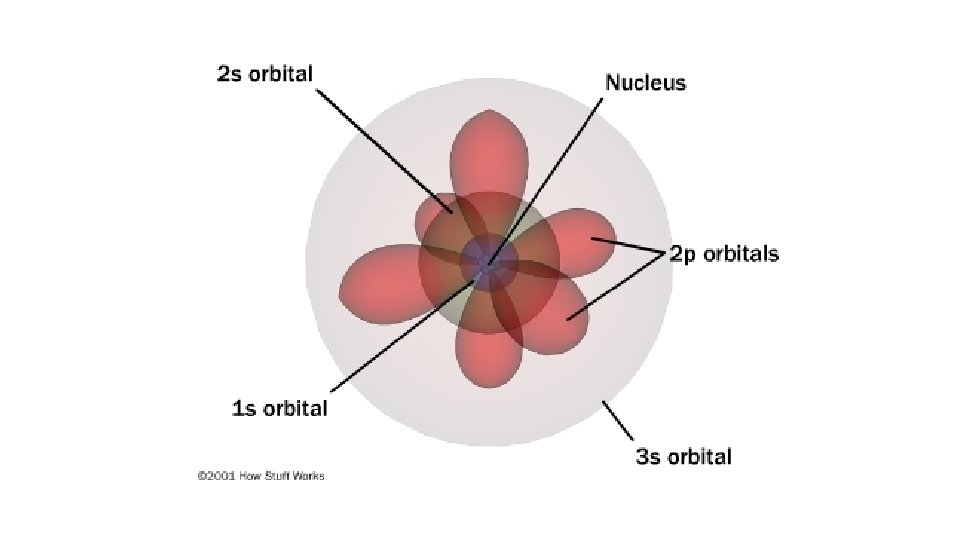
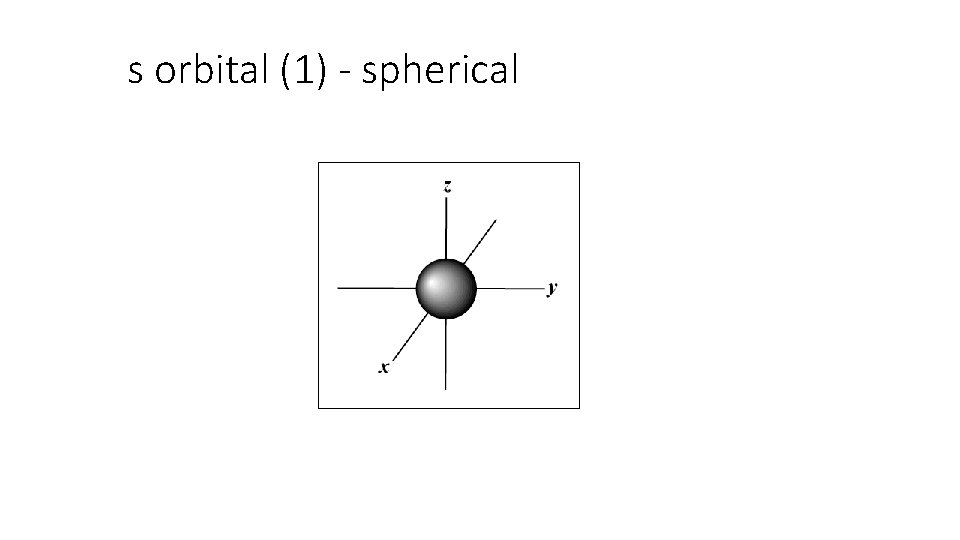
B. Atomic Orbitals • An atomic orbital is a region of space in which there is a high probability of finding an electron • assigned letters s, p, d or f (smart people do fine) • Energy sublevels correspond to a shape where the electron is likely to be found.

s orbital (1) - spherical
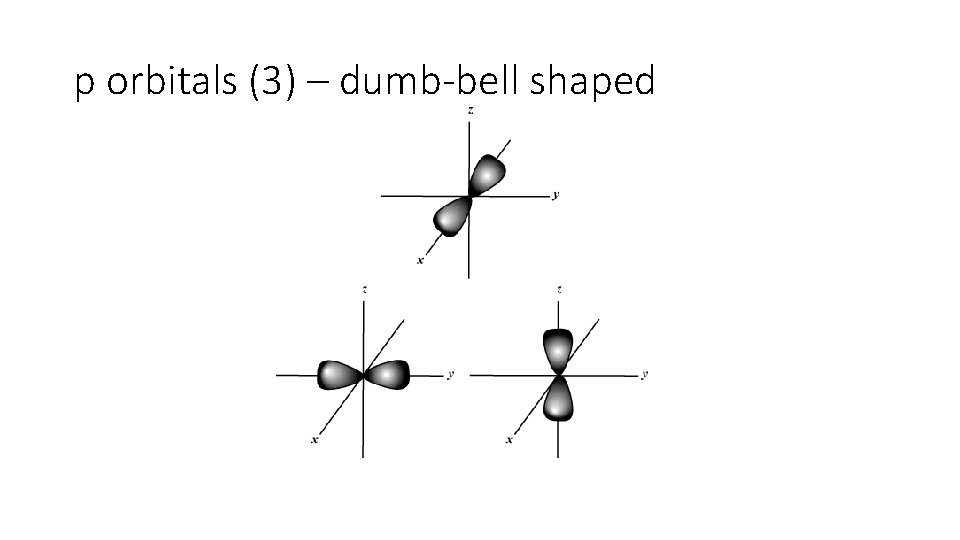
p orbitals (3) – dumb-bell shaped

d orbitals (5)

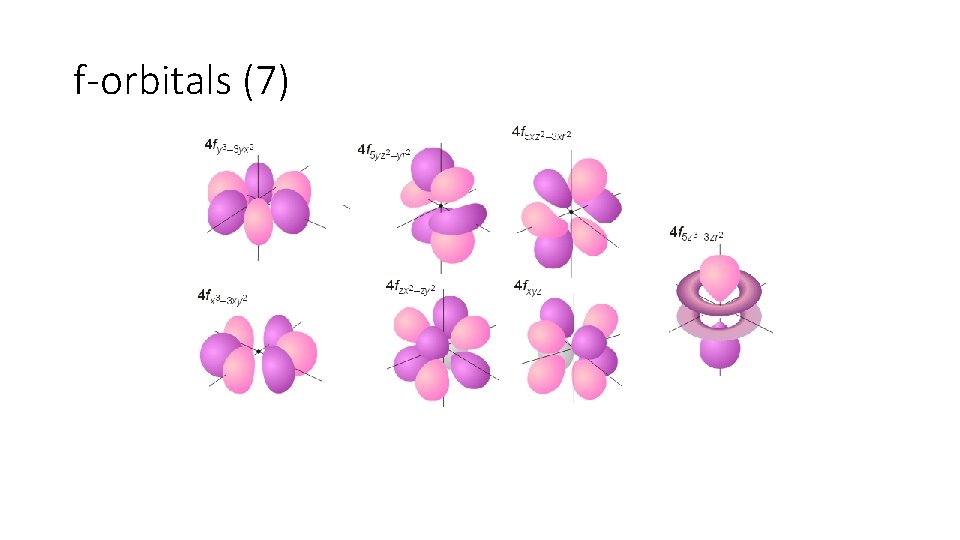
f-orbitals (7)

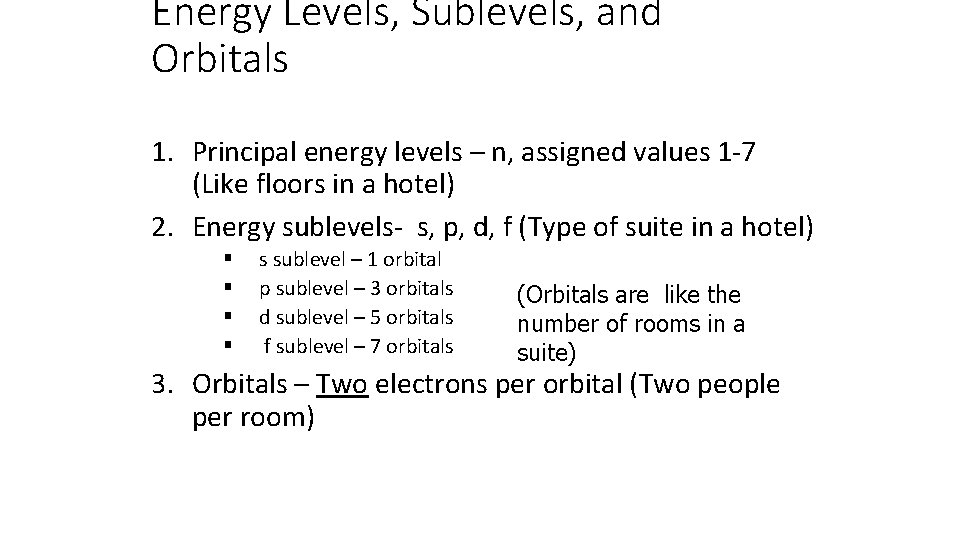
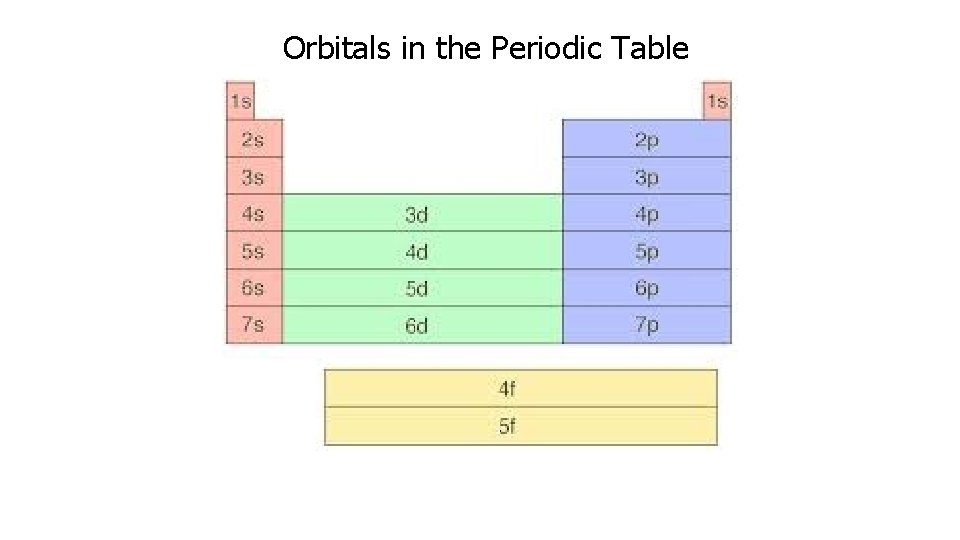
Energy Levels, Sublevels, and Orbitals 1. Principal energy levels – n, assigned values 1 -7 (Like floors in a hotel) 2. Energy sublevels- s, p, d, f (Type of suite in a hotel) § § s sublevel – 1 orbital p sublevel – 3 orbitals d sublevel – 5 orbitals f sublevel – 7 orbitals (Orbitals are like the number of rooms in a suite) 3. Orbitals – Two electrons per orbital (Two people per room)

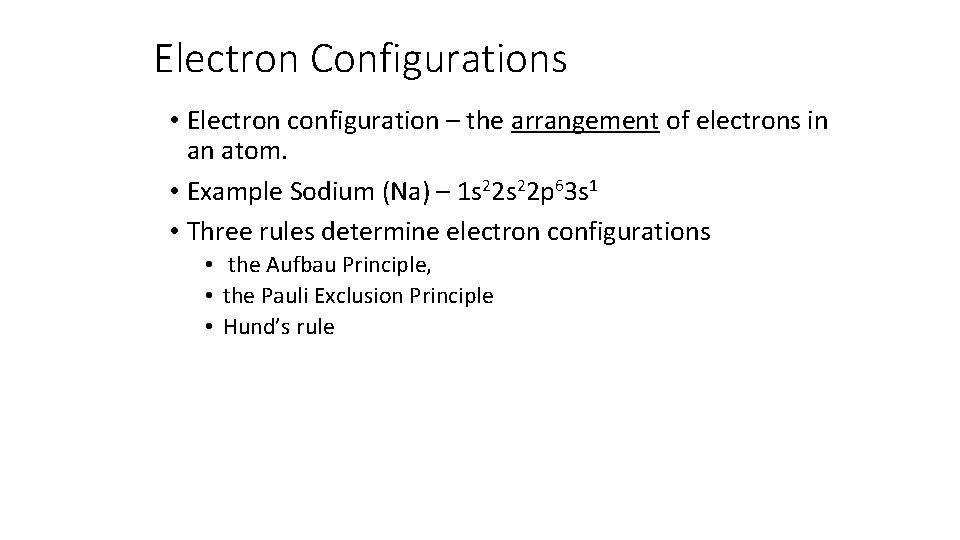
Electron Configurations • Electron configuration – the arrangement of electrons in an atom. • Example Sodium (Na) – 1 s 22 p 63 s 1 • Three rules determine electron configurations • the Aufbau Principle, • the Pauli Exclusion Principle • Hund’s rule

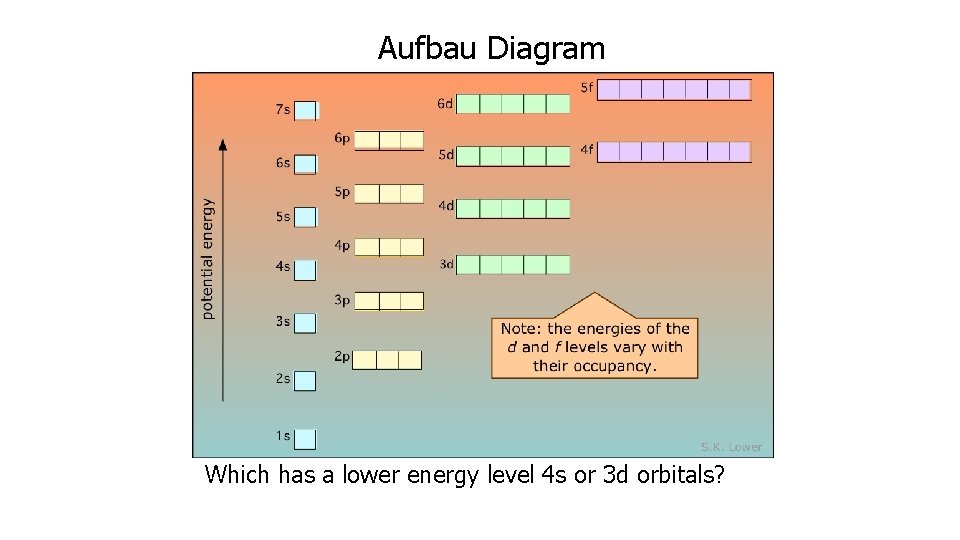
The Aufbau Principle • Each electron occupies the lowest energy orbital available • Like filling the hotel from the bottom up

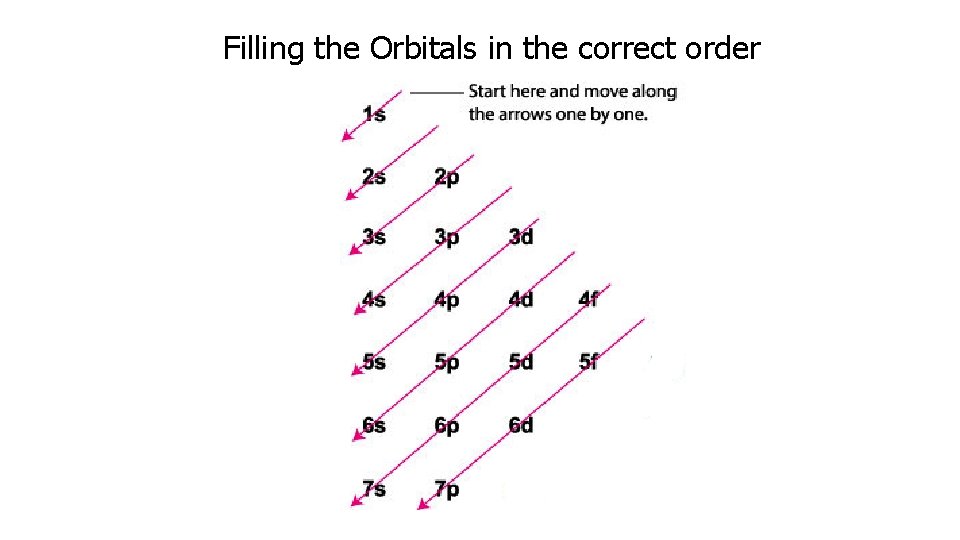
Aufbau Diagram Which has a lower energy level 4 s or 3 d orbitals?

Filling the Orbitals in the correct order

Orbitals in the Periodic Table