Atomic Structure The Periodic Table Atomic Theory Whats

Atomic Structure, The Periodic Table & Atomic Theory What’s it all about?

The Basic Atom �Smallest particle of an element that still has the element’s properties �Atomic Theory explains what atoms look like, and how and why they behave the way they do �Democritus (400 BC) proposed the existence of atoms (philosophy) �John Dalton (1800) was the first to have scientific evidence to support this � Considered the Father of Modern Atomic Theory

Dalton’s Atomic Theory �Dalton’s experiments showed him several things about the existence of atoms – he had 5 postulates �All matter is made of atoms �Atoms are small, indestructible spheres �Atoms of the same element are identical �Atoms always combine in compounds in whole number ratios �In chemical reactions atoms are combined, separated or rearranged

Dalton’s Model �Dalton’s idea of the atom �The Marble Model was a small sphere hence
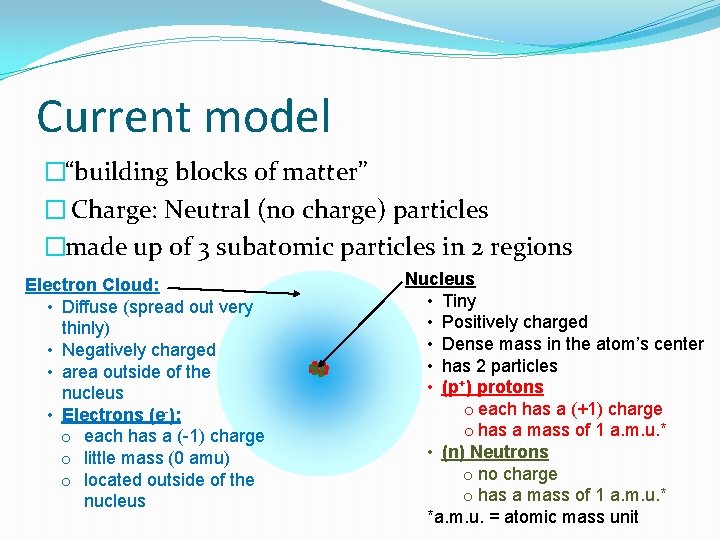
Current model �“building blocks of matter” � Charge: Neutral (no charge) particles �made up of 3 subatomic particles in 2 regions Electron Cloud: • Diffuse (spread out very thinly) • Negatively charged • area outside of the nucleus • Electrons (e-): o each has a (-1) charge o little mass (0 amu) o located outside of the nucleus Nucleus • Tiny • Positively charged • Dense mass in the atom’s center • has 2 particles • (p+) protons o each has a (+1) charge o has a mass of 1 a. m. u. * • (n) Neutrons o no charge o has a mass of 1 a. m. u. * *a. m. u. = atomic mass unit
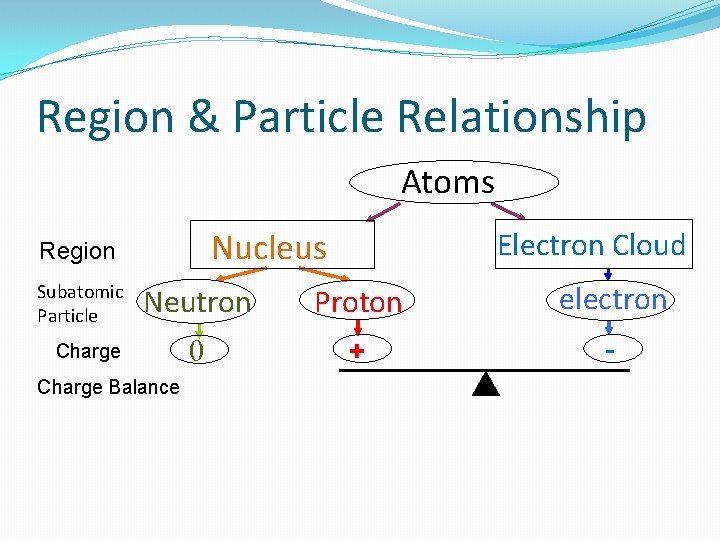
Region & Particle Relationship Atoms Nucleus Region Subatomic Particle Electron Cloud Neutron Proton electron 0 + - Charge Balance

Atomic Particles The Electron

The Electron– Fun Facts �Abbreviated e�Gives the electron cloud its charge �Gives the element its chemical properties �Structure: Lepton (fundamental particle not made of smaller particles) �Mass = 0 amu* (amu = atomic mass unit) �Not really 0 but 1/1200 -1/2000 th the mass of a proton) �Charge: negative (exactly balances the p+ positive charge) �Location: Electron Cloud
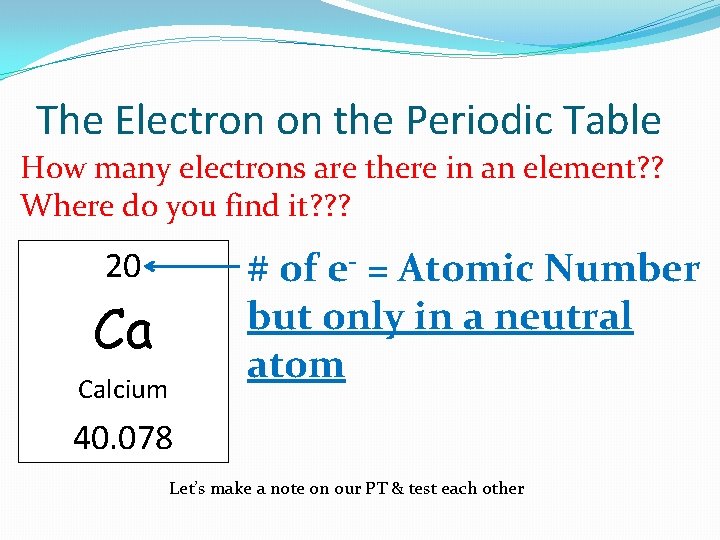
The Electron on the Periodic Table How many electrons are there in an element? ? Where do you find it? ? ? # of e- = Atomic Number but only in a neutral atom 20 Ca Calcium 40. 078 Let’s make a note on our PT & test each other

The Electron – History/Theory �Discovery #: 1 st subatomic particle discovered �Discovered by JJ Thomson in 1897 �JJ Made a CRT (cathode ray tube) and found that when he shot electricity through it a beam of light appeared �The beam of light was affected by magnetic & electric forces (does light do that? ? ) �He proposed that it wasn’t light but tiny, negatively charged particles he called electrons (btw: he thought the discovery was useless)
Thomson’s Model �The atom was �Neutral in charge so… � Most of the atom was a diffuse positively charged “goo” or matrix � Electrons were tiny particles that floated around in the “goo”. �Electrons were very tiny compared to the mass of the atom
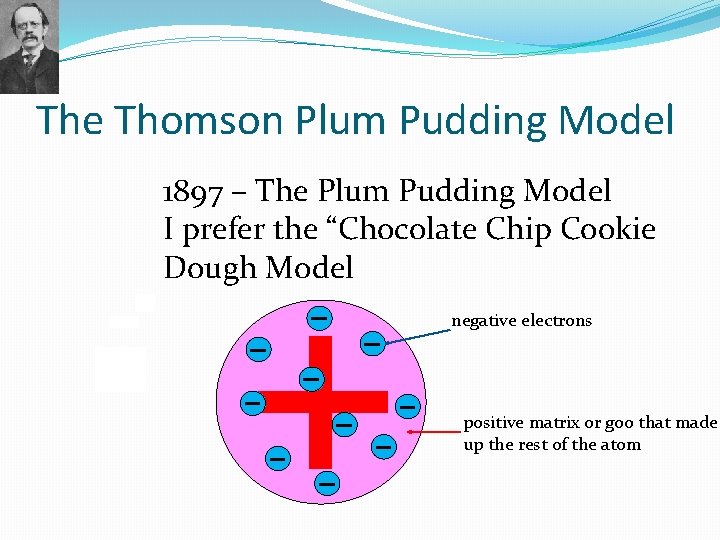
The Thomson Plum Pudding Model 1897 – The Plum Pudding Model I prefer the “Chocolate Chip Cookie Dough Model negative electrons positive matrix or goo that made up the rest of the atom

Atomic Particles The Proton

The Proton – Fun Facts �Abbreviated p+ �Gives the nucleus a positive charge �Gives the element its unique characteristics & identifies the element (# p + is unique to each element �Structure: Hadron (composite particle made of smaller particles) �Mass = 1 amu* �Charge: Positive �Location: Nucleus
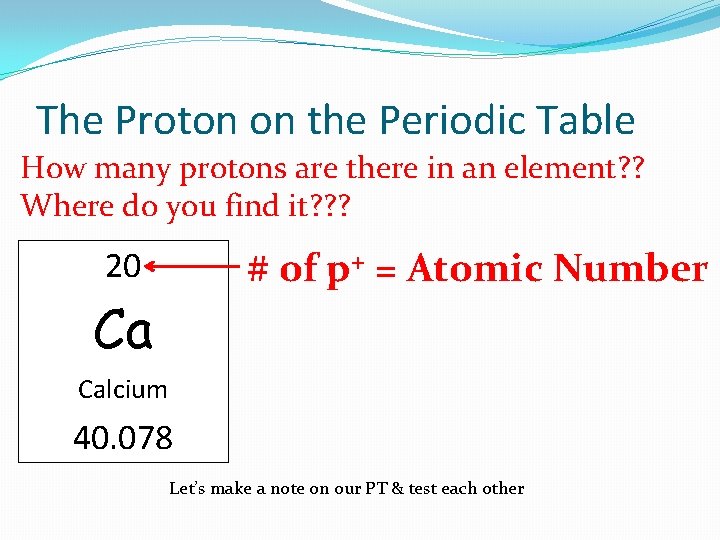
The Proton on the Periodic Table How many protons are there in an element? ? Where do you find it? ? ? # of p+ = Atomic Number 20 Ca Calcium 40. 078 Let’s make a note on our PT & test each other

The Proton – History/Theory �Discovery #: 2 nd subatomic particle discovered �Discovered by Ernest Rutherford in 1918 �Rutherford first proposed the positive nucleus in 1911 from the “Gold Foil Experiment” changing the model of the atom again �http: //www. mhhe. com/physsci/chemistry/essentialch emistry/flash/ruther 14. swf

Rutherford’s Models �The nucleus was �Very dense �Had most of the mass of the atom �Was positive �Very, very tiny compared to the entire atom �Atoms are mostly empty space �The electrons move around the outside of the nucleus �Rutherford model – nuclear model – kind of like a plasma ball w/ a tiny positive nucleus and electrons floating around outside it �Discovered the proton later in the first nuclear reaction �How tiny? 400 x tinier than this – and that’s only the nucleus!!! Protons are smaller yet!!!!

The Rutherford (Nuclear) Models 1911 Model with just a positive nucleus in the center of the electrons 1918 Model with protons making up the nucleus

Atomic Particles The Neutron

The Neutron – Fun Facts �Abbreviated n 0 �Adds mass to the nucleus �Helps hold the nucleus together (prevents proton repulsion from destroying the nucleus) �Structure: Hadron (composite particle made of smaller particles) �Mass = 1 amu* �Charge: Neutral (no charge) �Location: Nucleus
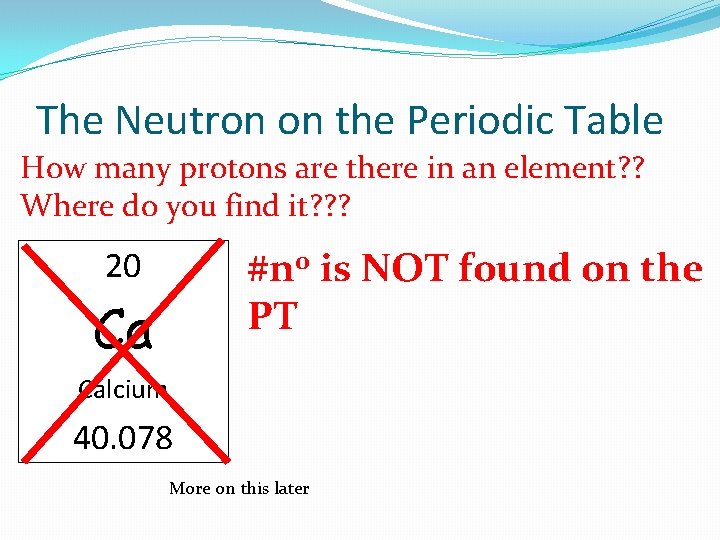
The Neutron on the Periodic Table How many protons are there in an element? ? Where do you find it? ? ? #n 0 is NOT found on the PT 20 Ca Calcium 40. 078 More on this later

The Neutron – History/Theory �Discovery #: 3 rd subatomic particle discovered �Discovered by James Chadwick (also a student of JJ Thomson) in 1936 – (Rutherford said it should be their, Chadwick gave them evidence) �Chadwick used a CRT & produced a beam of neutral particles that knocked protons out of paraffin wax �https: //www. youtube. com/watch? v=Hnm. EI 94 URK 8 �Led to the development of the atomic bomb
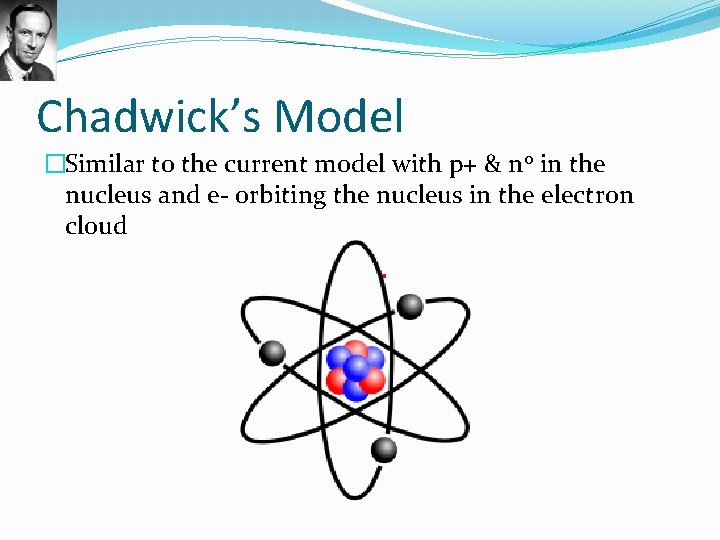
Chadwick’s Model �Similar to the current model with p+ & n 0 in the nucleus and e- orbiting the nucleus in the electron cloud
- Slides: 23