Atomic Structure Subatomic Particles Nuclear Protons Neutrons Nonnuclearelectrons
Atomic Structure

Subatomic Particles Nuclear Protons Neutrons Non-nuclear—electrons

Niels Bohr Theory Electrons orbit the nucleus in specific energy levels, like tracks. The energy levels are certain distances from the nucleus. These levels keep the electrons from crashing into the nucleus (positive and negative attract!). Electrons can move levels by absorbing and losing energy in the form of light.
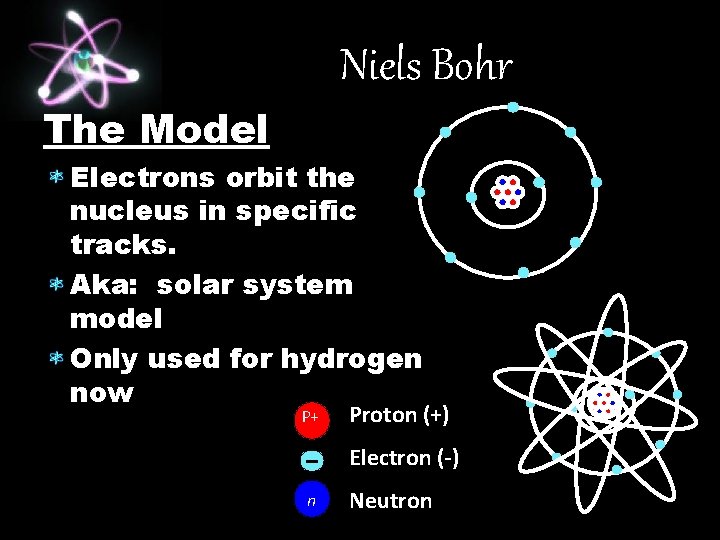
Niels Bohr The Model Electrons orbit the nucleus in specific tracks. Aka: solar system model Only used for hydrogen now P+ Proton (+) Electron (-) n Neutron

Protons Found in the nucleus of the atom Have a positive charge Are the same size as a neutron and much bigger than electrons Has a mass about the same as a neutron

Neutrons Found in the nucleus of the atom Have no charge (is neutral) Are the same size as a proton and much bigger than electrons Has a mass about the same as a proton

Electrons Found outside the nucleus of the atom Have a negative charge Is extremely small, much smaller than protons and neutrons Have almost no mass
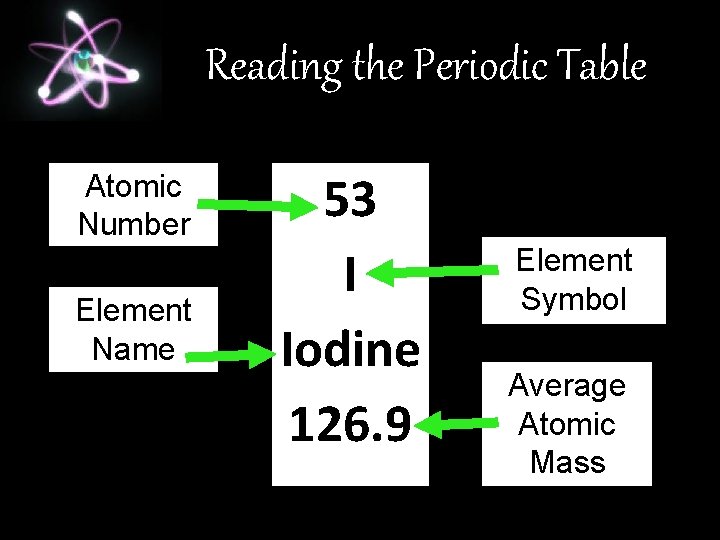
Reading the Periodic Table Atomic Number Element Name 53 I Iodine 126. 9 Element Symbol Average Atomic Mass
Atomic Number Defines or identifies the element Is the same as the number of protons in an atom of that element All elements have a different atomic number (no repeats)
Average Atomic Mass Isotopes are two atoms of the same element that have a different number of neutrons and a different mass number Isotopes are like identical twins with different hair cuts. Is the average mass of all the isotopes of that element

Mass Number Is the sum of the protons and neutrons for an atom Can be found in many ways: After the isotope’s name The top number in the isotope’s symbol Adding the number of protons and neutrons Rounding the average atomic mass to a whole number (this is only if 1 -3 are not available)
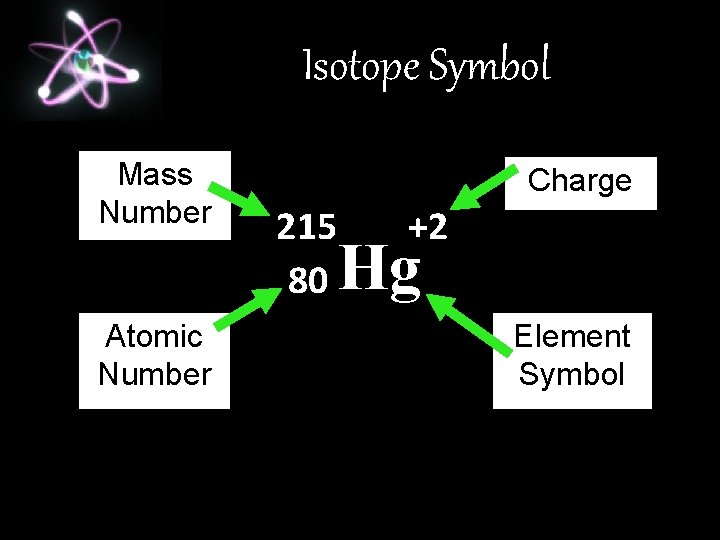
Isotope Symbol Mass Number Atomic Number Charge 215 80 +2 Hg Element Symbol
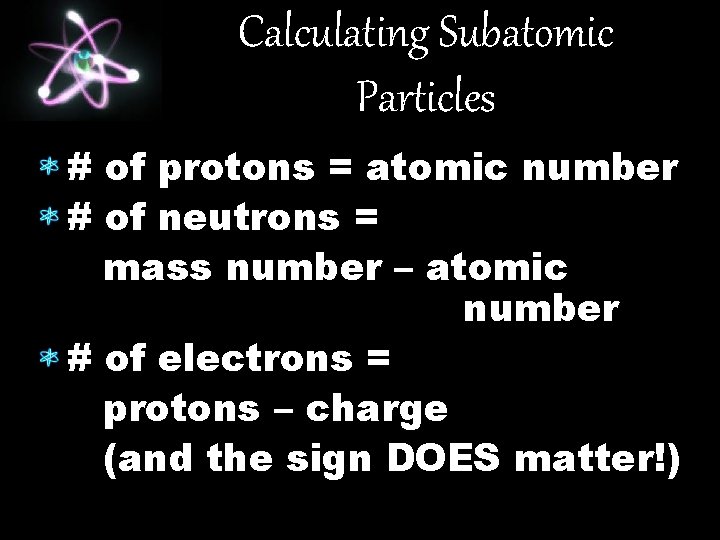
Calculating Subatomic Particles # of protons = atomic number # of neutrons = mass number – atomic number # of electrons = protons – charge (and the sign DOES matter!)

Think it through. . . Why should we use isotope symbols to display information about an atom?
- Slides: 14