Atomic Structure Revision Parts of a molecule Proton

- Slides: 10

Atomic Structure Revision!

Parts of a molecule Proton - positively charged - inside nucleus Neutron - neutrally charged - inside nucleus Electron - negatively charged - orbits nucleus Electrons are 2000 times smaller than protons and neutrons and are therefore not counted when calculating mass.

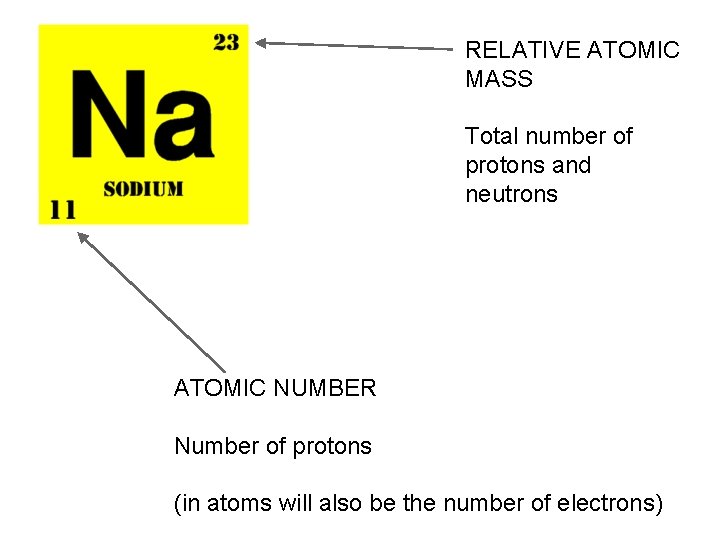
RELATIVE ATOMIC MASS Total number of protons and neutrons ATOMIC NUMBER Number of protons (in atoms will also be the number of electrons)

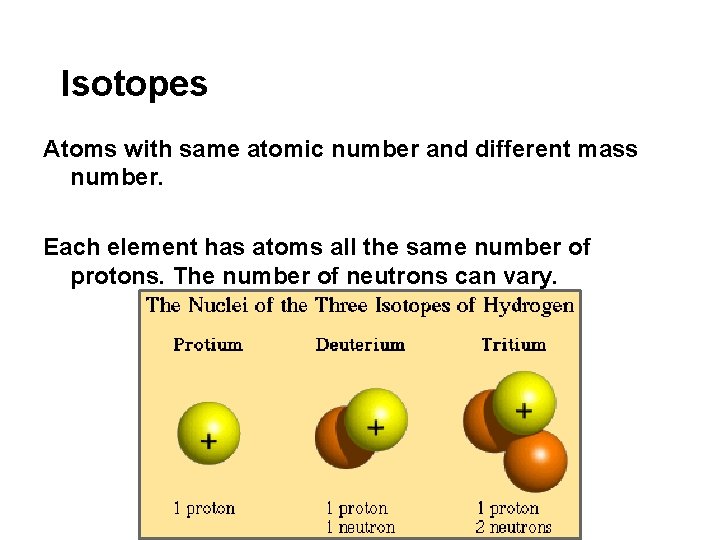
Isotopes Atoms with same atomic number and different mass number. Each element has atoms all the same number of protons. The number of neutrons can vary.

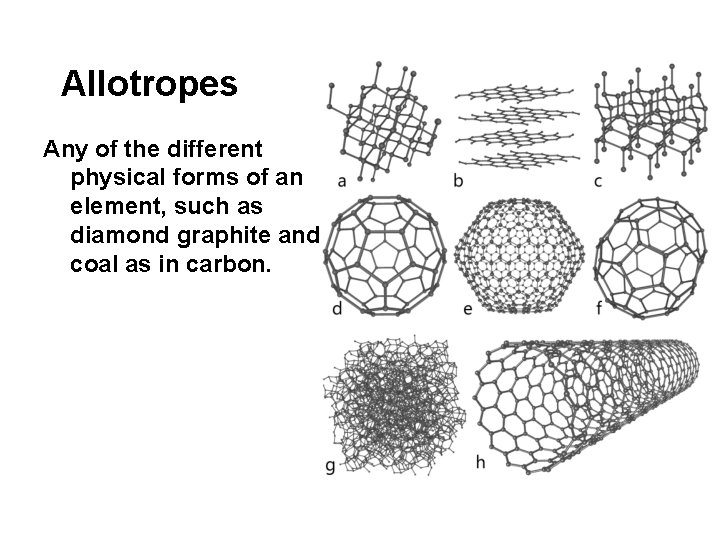
Allotropes Any of the different physical forms of an element, such as diamond graphite and coal as in carbon.

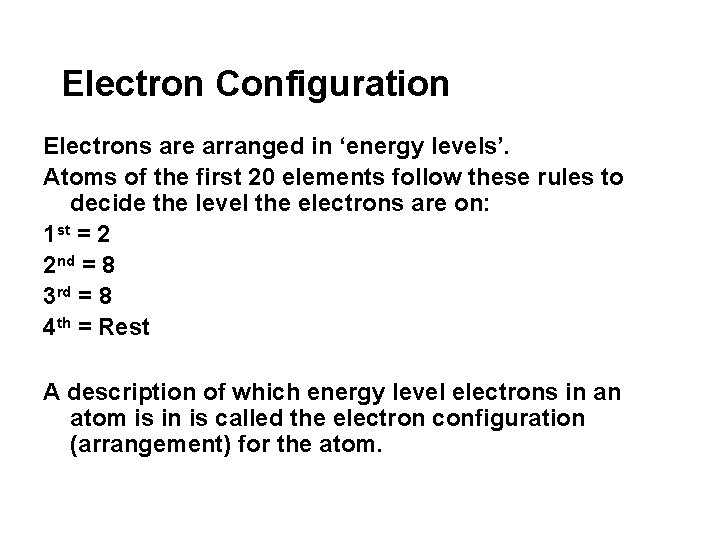
Electron Configuration Electrons are arranged in ‘energy levels’. Atoms of the first 20 elements follow these rules to decide the level the electrons are on: 1 st = 2 2 nd = 8 3 rd = 8 4 th = Rest A description of which energy level electrons in an atom is in is called the electron configuration (arrangement) for the atom.

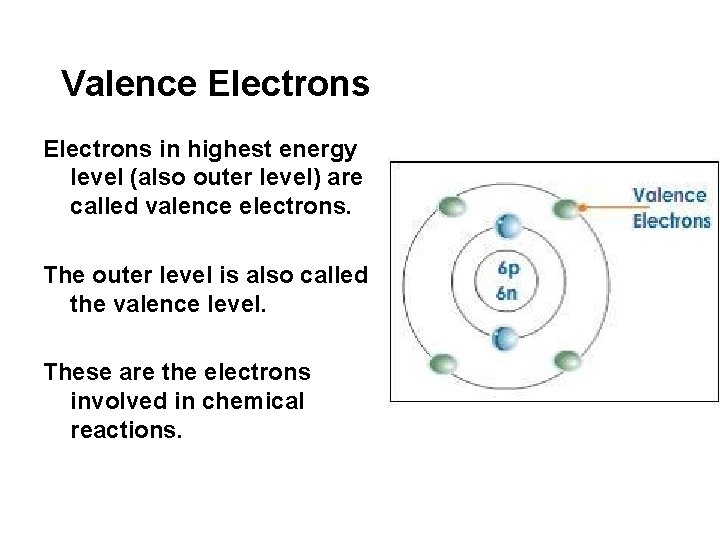
Valence Electrons in highest energy level (also outer level) are called valence electrons. The outer level is also called the valence level. These are the electrons involved in chemical reactions.

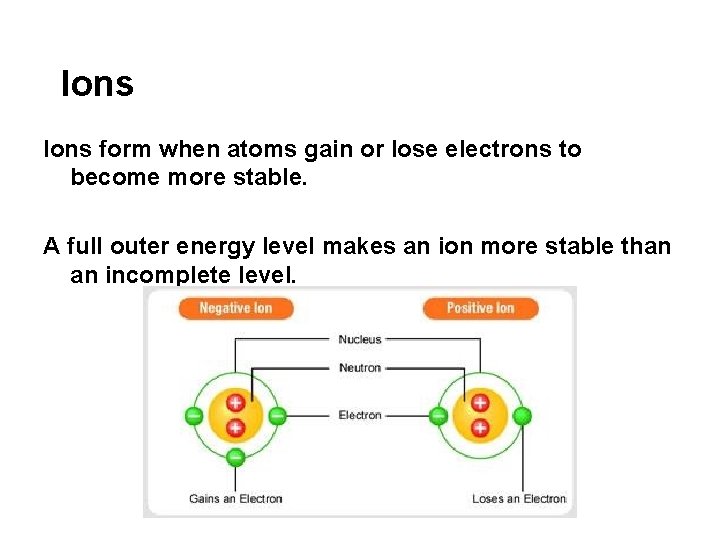
Ions form when atoms gain or lose electrons to become more stable. A full outer energy level makes an ion more stable than an incomplete level.

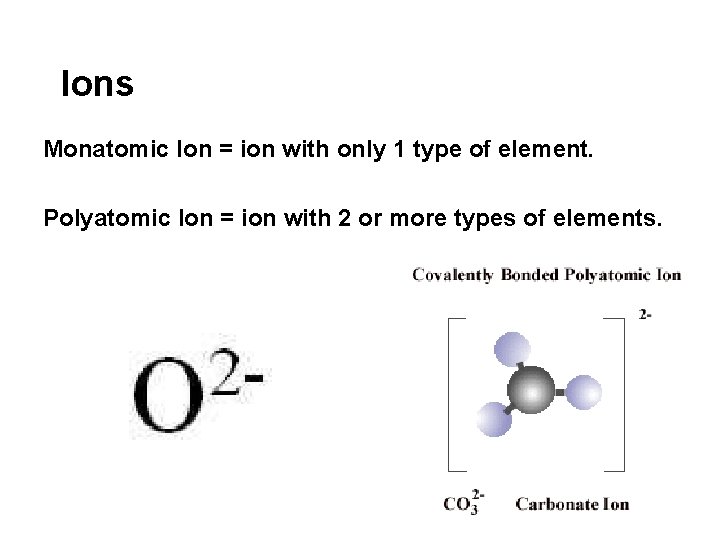
Ions Monatomic Ion = ion with only 1 type of element. Polyatomic Ion = ion with 2 or more types of elements.

Forming Molecules A chemical bond is a force holding 2 atoms together. A bond forms to produce a full outer energy level for both/all atoms. Atoms combine together to gain a more stable structure. Ionic Bonding – where an atom gives up its valence electrons completely. Covalent Bonding – an electron pair is shared between 2 atoms.