Atomic structure Protons mass 1 charge Neutrons mass

- Slides: 2

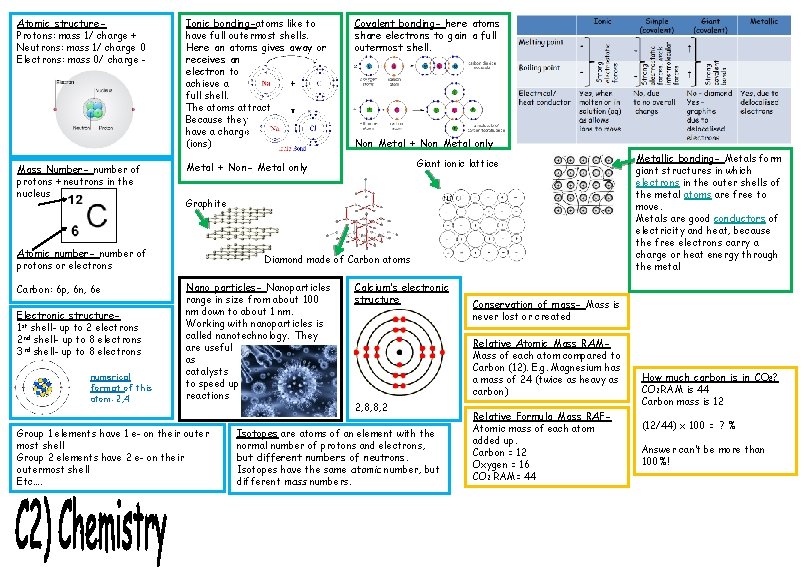
Atomic structure. Protons: mass 1/ charge + Neutrons: mass 1/ charge 0 Electrons: mass 0/ charge - Mass Number- number of protons + neutrons in the nucleus Ionic bonding-atoms like to have full outermost shells. Here an atoms gives away or receives an electron to achieve a full shell. The atoms attract Because they have a charge (ions) Electronic structure 1 st shell- up to 2 electrons 2 nd shell- up to 8 electrons 3 rd shell- up to 8 electrons numerical format of this atom: 2, 4 Non Metal + Non Metal only Giant ionic lattice Metal + Non- Metal only Graphite Atomic number- number of protons or electrons Carbon: 6 p, 6 n, 6 e Covalent bonding- here atoms share electrons to gain a full outermost shell. Diamond made of Carbon atoms Nano particles- Nanoparticles range in size from about 100 nm down to about 1 nm. Working with nanoparticles is called nanotechnology. They are useful as catalysts to speed up reactions Group 1 elements have 1 e- on their outer most shell Group 2 elements have 2 e- on their outermost shell Etc…. Calcium’s electronic structure Conservation of mass- Mass is never lost or created Relative Atomic Mass RAMMass of each atom compared to Carbon (12). E. g. Magnesium has a mass of 24 (twice as heavy as carbon) 2, 8, 8, 2 Isotopes are atoms of an element with the normal number of protons and electrons, but different numbers of neutrons. Isotopes have the same atomic number, but different mass numbers. Metallic bonding- Metals form giant structures in which electrons in the outer shells of the metal atoms are free to move. Metals are good conductors of electricity and heat, because the free electrons carry a charge or heat energy through the metal Relative Formula Mass RAFAtomic mass of each atom added up. Carbon = 12 Oxygen = 16 CO 2 RAM= 44 How much carbon is in CO 2? CO 2 RAM is 44 Carbon mass is 12 (12/44) x 100 = ? % Answer can’t be more than 100%!

Increased temperature = increased rate of reaction • the reactant particles move more quickly • more particles have the activation energy or greater • the particles collide more often, and more of the collisions result in a reaction • the rate of reaction increases Increasing surface area= increased rate of reaction • its surface area is increased • more particles are exposed to the other reactant • there is a greater chance of the particles colliding • the rate of reaction increases Catalysts increase the rate of reaction without being used up. They do this by lowering the activation energy needed. With a catalyst, more collisions result in a reaction, so the rate of reaction increases. Different reactions need different catalysts. Exothermic reactions= gives off heat (feels hot) Endothermic reaction= takes in heat (feels cold) • Increasing concentration = increased rate of reaction there are more reactant particles in the same volume • there is a greater chance of the particles colliding • the rate of reaction increases Reversible reactions- you can get back to the original reactants e. g. ammonium chloride ammonia + hydrogen chloride Electrolysis is the process by which ionic substances are broken down into simpler substances using electricity. During electrolysis, metals and gases may form at the electrodes The Haber process- making ammonia gas Nitrogen and hydrogen will react together under these conditions: • a high temperature - about 450ºC • a high pressure - about 200 atmospheres (200 times normal pressure) • an iron catalyst N 2(g) + 3 H 2(g) 2 NH 3(g) Splitting up Na. Cl 2 Increasing pressure = increased rate of reaction. • Because there are more reactant particles in the same volume • there is a greater chance of the particles colliding • the rate of reaction increases Acids- substances with a p. H of 1 6 Neutral- substance with a p. H of 7 Alkali/ Basesubstance with a p. H of 8 14 Neutralisation. Acid + Alkali Salt + Water (Neutral) Acid + Metal Salt + Hydrogen gas Acid + metal carbonate salt + water + carbon dioxide