Atomic Structure of Materials Bonding between Atoms and
Atomic Structure of Materials • Bonding between Atoms and Molecules • Atoms are held together in molecules by various types of bonds • Two types of bonding: Primary and Secondary • Primary Bonds are know to have strong atom -to atom attractions that involve the exchange of valence electrons. • Examples of primary bonds; ionic, covalent and metallic

Atomic Structure of Materials • Secondary bonds- are generally associated with attraction between molecules • There are no transfer of or sharing of electrons in secondary bonding, and these bonds are therefore weaker than primary bonds • Secondary bonds involves attraction of forces between molecules • There are three forms of secondary bonding: dipole forces, London forces and hydrogen bonding
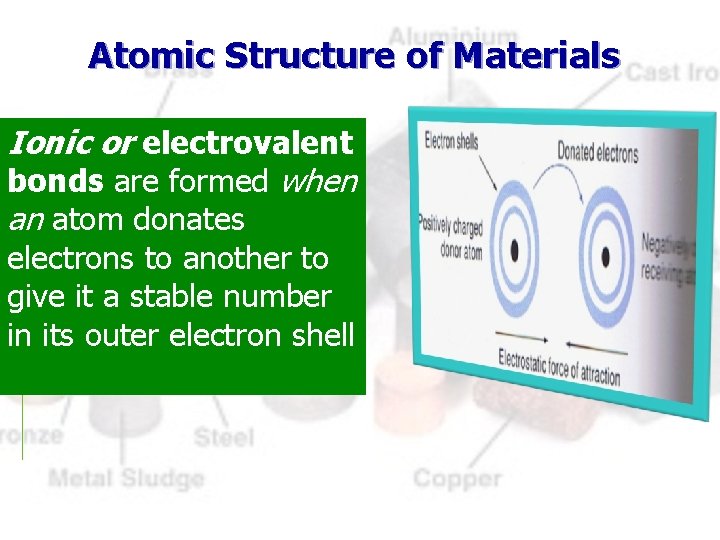
Atomic Structure of Materials Ionic or electrovalent bonds are formed when an atom donates electrons to another to give it a stable number in its outer electron shell
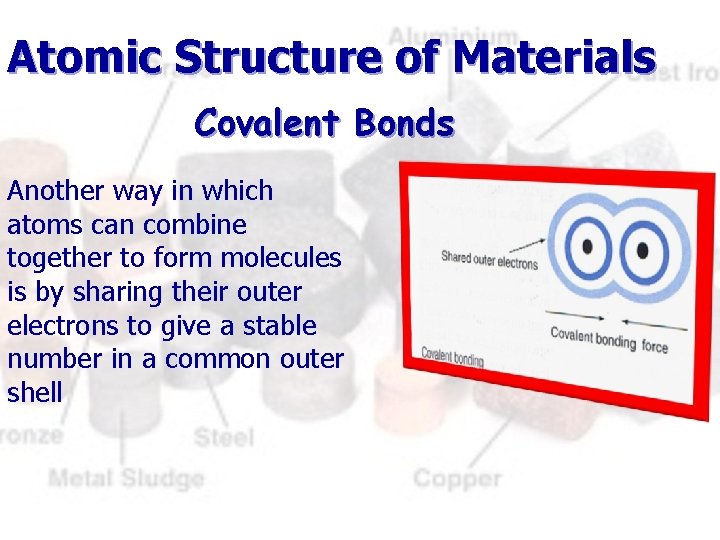
Atomic Structure of Materials Covalent Bonds Another way in which atoms can combine together to form molecules is by sharing their outer electrons to give a stable number in a common outer shell
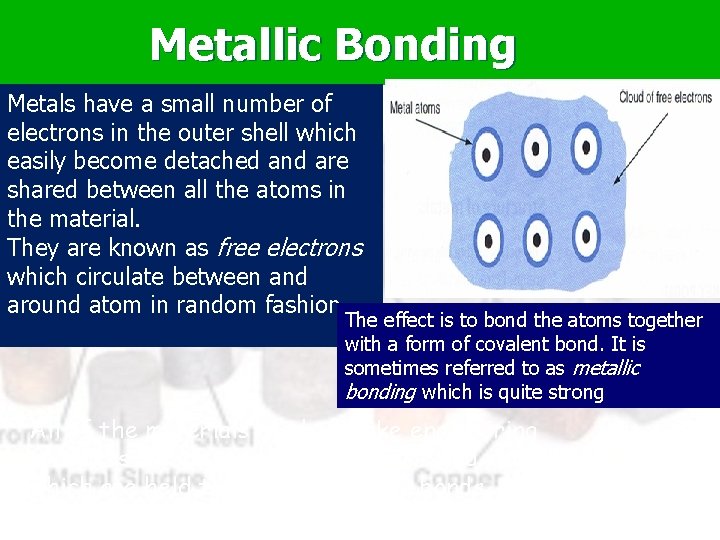
Metallic Bonding Metals have a small number of electrons in the outer shell which easily become detached and are shared between all the atoms in the material. They are known as free electrons which circulate between and around atom in random fashion. The effect is to bond the atoms together with a form of covalent bond. It is sometimes referred to as metallic bonding which is quite strong All of the materials used to make engineering products are thus made up of atoms and molecules which are held together by strong bonds. And they are all different

Structure of Materials The structure of materials can be classified by the general magnitude of various features being considered • Atomic Structure • The structure of atoms is important to Materials Engineers because it influences the way atoms are bonded together which in turn helps us to categorize the materials that they form. • The atomic structure and bonding also allows us to formulate some general conclusions on the mechanical and physical properties of the material.

https: //www. youtube. com/watc h? v=Vt. RP 6 RDw. QIg
Structure of Materials • Atomic structure- which includes features that cannot be seen, such as the types of bonding between the atoms, and the way the atoms are arranged. • Microstructure- which includes features that can be seen using a microscope, but seldom with the naked eye. • Macrostructure- which includes features that can be seen with the naked eye)
Metallic Structures • Most engineering materials such as metals and ceramics are made up of crystals. These crystals consist of atoms which are packed into a repeating three dimensional structure. Metals form three main crystaline structures. • Body centred cubic (b. c. c), • face centred cubic (f. c. c) and • Hexagonally close packed (h. c. p).

Body-Centred Cubic (BCC) Structure • The body-centred cubic unit cell has atoms at each of the eight corners of a cube (like the cubic unit cell) plus one atom in the centre of the cube (left image below). • Examples of the bcc structure lithium, sodium, potassium, chromium, barium, vanadium, alpha-iron and tungsten.
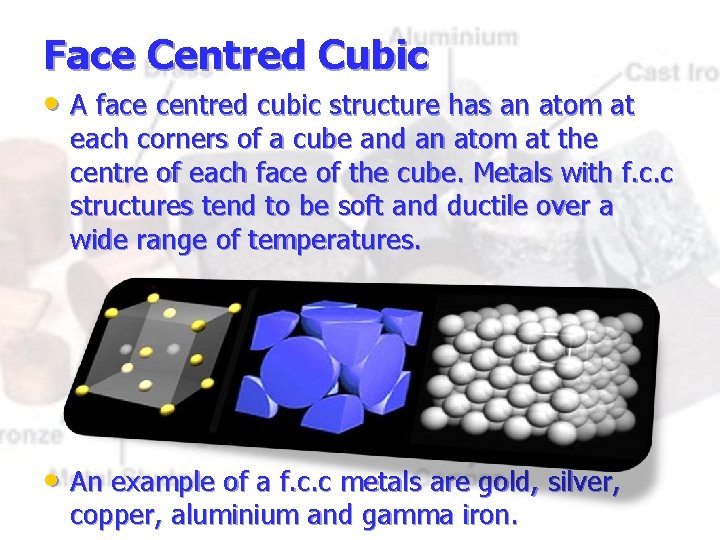
Face Centred Cubic • A face centred cubic structure has an atom at each corners of a cube and an atom at the centre of each face of the cube. Metals with f. c. c structures tend to be soft and ductile over a wide range of temperatures. • An example of a f. c. c metals are gold, silver, copper, aluminium and gamma iron.

Hexagonally Closed Packed • Hexagonally closed packed structures have an atom at the corners of an imaginary hexagonal prism and an atom at the centre of each hexagonal face and tree atoms in the centre forming a 'prism'. • some examples include beryllium, cadmium, magnesium, titanium, zinc and zirconium. These metals are relatively brittle such as cobalt and magnesium.

Shrinkage Most materials contract or shrink during solidification and cooling. Shrinkage is the result of: • Contraction of the liquid as it cools prior to its solidification • Contraction during phase change from a liquid to solid • Contraction of the solid as it continues to cool to ambient temperature.
Crystallization is the transformation from the liquid to the solid state and occurs in two stages as nuclei formation and crystal growth.
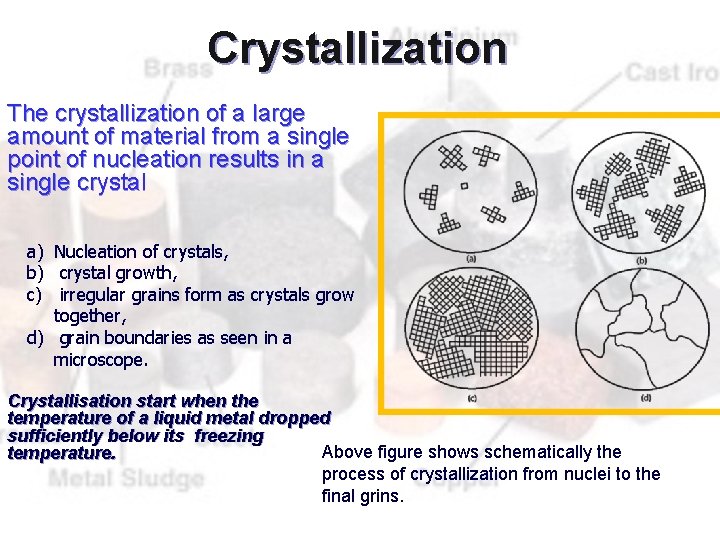
Crystallization The crystallization of a large amount of material from a single point of nucleation results in a single crystal a) Nucleation of crystals, b) crystal growth, c) irregular grains form as crystals grow together, d) grain boundaries as seen in a microscope. Crystallisation start when the temperature of a liquid metal dropped sufficiently below its freezing Above figure shows schematically the temperature. process of crystallization from nuclei to the final grins.
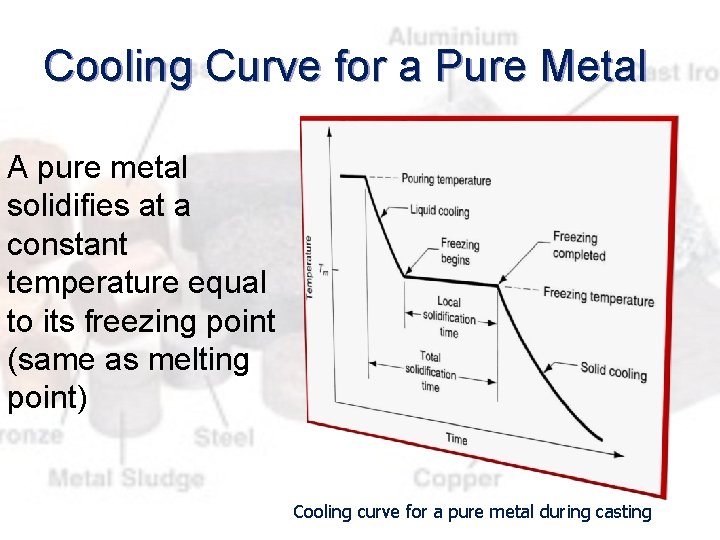
Cooling Curve for a Pure Metal A pure metal solidifies at a constant temperature equal to its freezing point (same as melting point) Cooling curve for a pure metal during casting
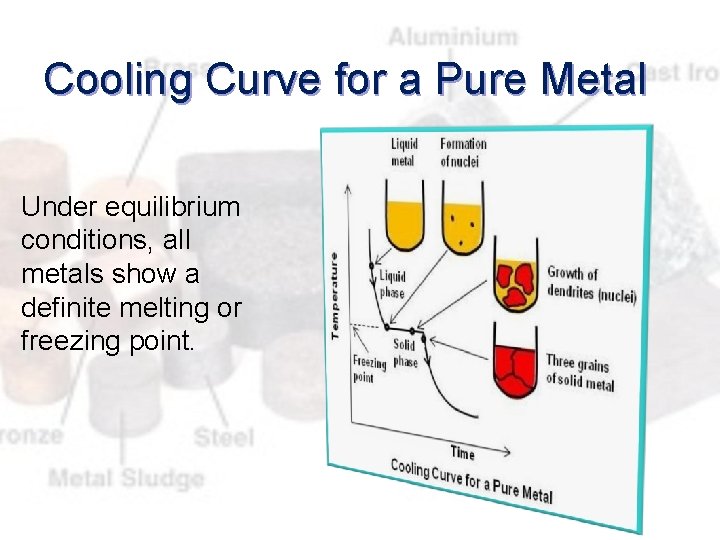
Cooling Curve for a Pure Metal Under equilibrium conditions, all metals show a definite melting or freezing point.
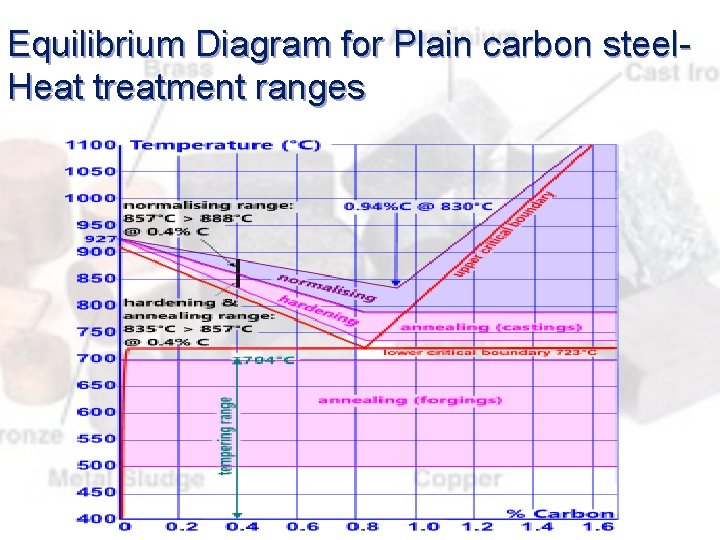
Equilibrium Diagram for Plain carbon steel. Heat treatment ranges

Why are metals tested ? • • Ensure quality Test properties Prevent failure in use Make informed choices in using materials Factor of Safety is the ratio comparing the actual stress on a material and the safe useable stress.
Two forms of testing • Mechanical tests – the material may be physically tested to destruction. Will normally specify a value for properties such as strength, hardness, toughness, etc. • Non-destructive tests (NDT) – samples or finished articles are tested before being used.
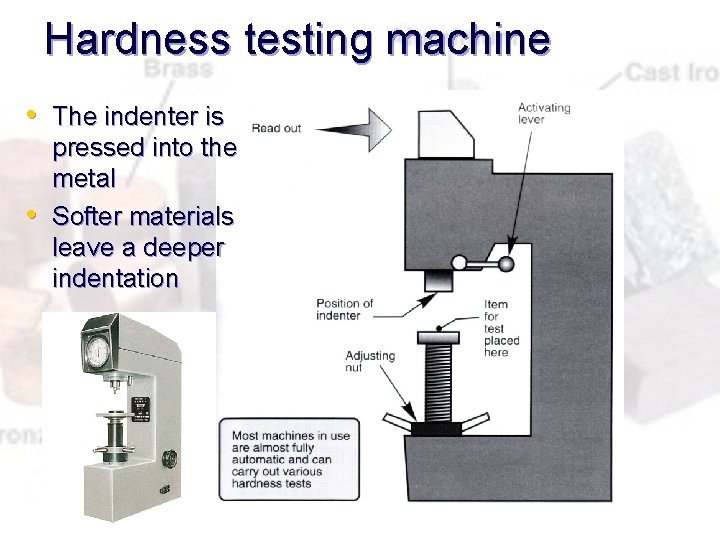
Hardness testing machine • The indenter is • pressed into the metal Softer materials leave a deeper indentation
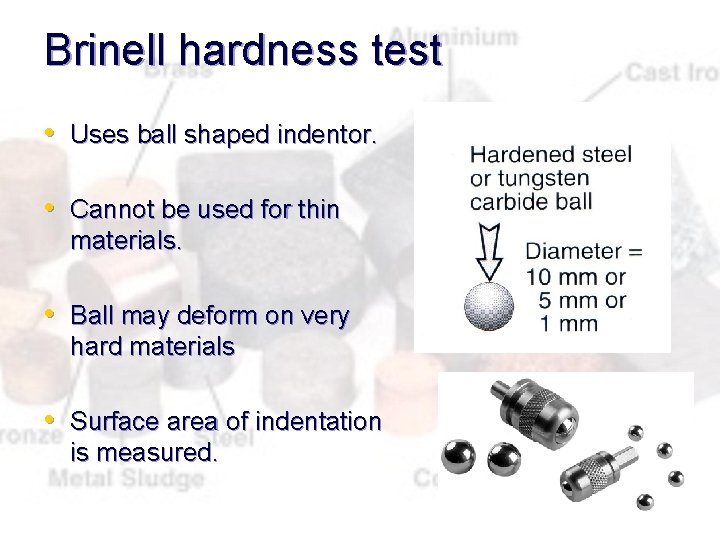
Brinell hardness test • Uses ball shaped indentor. • Cannot be used for thin materials. • Ball may deform on very hard materials • Surface area of indentation is measured.

Brinell hardness test Hardness test can also be used to obtained • Estimate of tensile strength in carbon steel • Can also helps to indicate work hardening capacity in carbon steel • To indicate machinability in carbon steel

Vickers hardness test • Uses square shaped pyramid indentor. • Accurate results. • Measures length of diagonal on indentation. • Usually used on very hard materials

Rockwell hardness tests • Gives direct reading. • Rockwell B (ball) used for soft materials. • Rockwell C (cone) uses diamond cone for hard materials. • Flexible, quick and easy to use.
Polymer structure • The term polymer is used to indicate that a compound consists of many repeated structural units, • The prefix ‘poly’ means many • Each repeating structural units in the compound is called a monomer or mer • Polymer materials include plastics and rubbers whose atoms are arranged in long molecular chains known as polymers.

• Polymerisation is the process by which these smaller units are joined together to create a large chain. Polymer include such materials like rubber and plastics. • Plastics are subdivided into thermoplastics, thermosetting plastics and rubbers • Thermoset plastics- are rigid plastics, resistant to higher temperatures than thermoplastics. Once set, a thermoset plastic cannot be remolded. There are several common thermoset plastics:
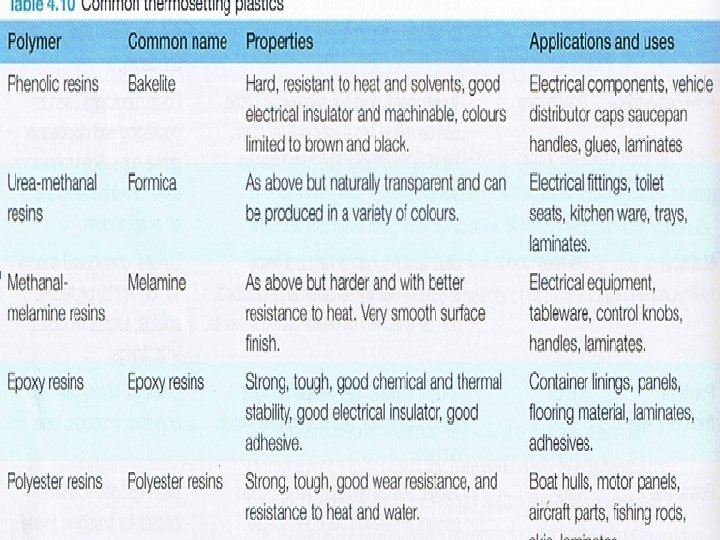
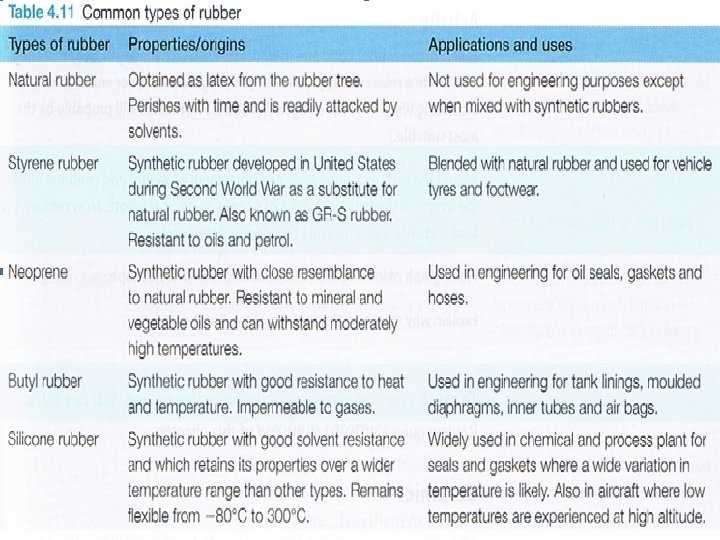

Thermoplastics become soft when heated. They can be easily moulded and remoulded without significant degrading. Thermoplastics consist of long molecular chains with no regular structure (or very little regular structure). base unit • Rubbers • The polymers in rubbers are known as elastomers. They are longer and tend to be more complex than other polymers. Elastomers can made up of more than 40000 atoms and when the material is unloaded, they are intertwined and folded over each other in a random

Last task of the session • What is a polymer?
- Slides: 31