Atomic Structure Matter anything that has a mass






- Slides: 10

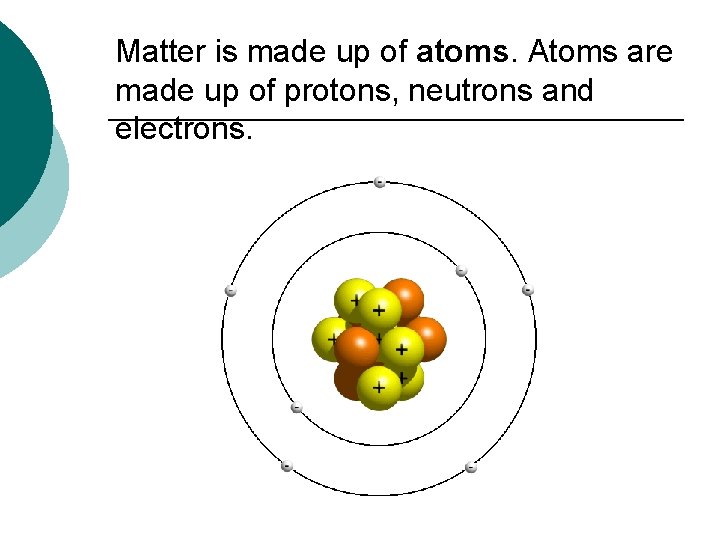
Atomic Structure Matter: anything that has a mass BUT…. What is matter made up of? AND Where did matter come from?

Matter is made up of atoms. Atoms are made up of protons, neutrons and electrons.

Hypothesis – First Models Of Matter There were many scientist that contribute to the development of the first models of matter 1. Aristotle 4. J. J. Thomson 2. Democritus 5. Rutherford 3. John Dalton 6. Bohr

Aristotle: Philosopher 300 BC (Greek) HE PROPOSED THAT: q All matter was continuous –no matter how small a substance was, the original properties were the same. q Matter was made of the four basic elements fire, water, air, earth. q Did not know about atoms.

Democritus: Philosopher 300 BC (Greek) HE PROPOSED THAT: q Matter was discontinuous q Matter can be broken into smaller particles q Defined the term atom

First Models • Despite Democritus’ accuracy, Aristotle’s ideas were more widely accepted. • Neither man was a scientist and neither did any experiments. • They were both thinkers and came up with ideas to explain what they saw in the world around them.

Developed by John Dalton ¡ ¡ He is known as the father of the Atomic Theory. He formulated four main points concerning atoms: Memorize 1) All matter is composed of tiny indivisible particle called atoms. 2) All atoms of the same element are identical (same). 3) Atoms of different elements are different. 4) During a chemical reaction atoms combine to form new products.

Dalton’s Atomic Model Even today, basic principles of his theory are still true. ¡ His model was modified to explain new observations of later chemists ¡

WHO SAID THE FOLLOWING? “Matter “All matter can be is broken made of into smaller earth, fire, particles” air or water” “No“Atoms matter of how small a substance different was, the original elements are properties different are the same”

ØOriginal Theories • Discontinuous (Democritis) – spaces between particles • Continuous (Aristotle) – no spaces between particles. Four elements – fire, water, earth & air. • Dalton’s Theory – memorize 4 main points