Atomic Structure JONATHAN SEIBERT PNHS PHYSICAL SCIENCE Greek
Atomic Structure JONATHAN SEIBERT PNHS – PHYSICAL SCIENCE
Greek Idea Democritus and Leucippus Matter is made up of solid indivisible particles

John Dalton First to present a written theory. If you want credit, you have to be able to write. one type of atom for each element
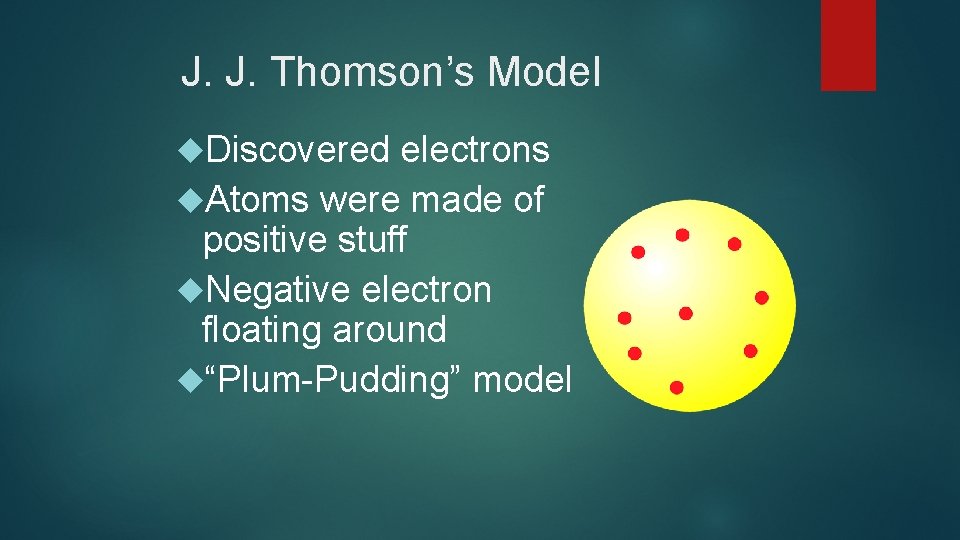
J. J. Thomson’s Model Discovered electrons Atoms were made of positive stuff Negative electron floating around “Plum-Pudding” model

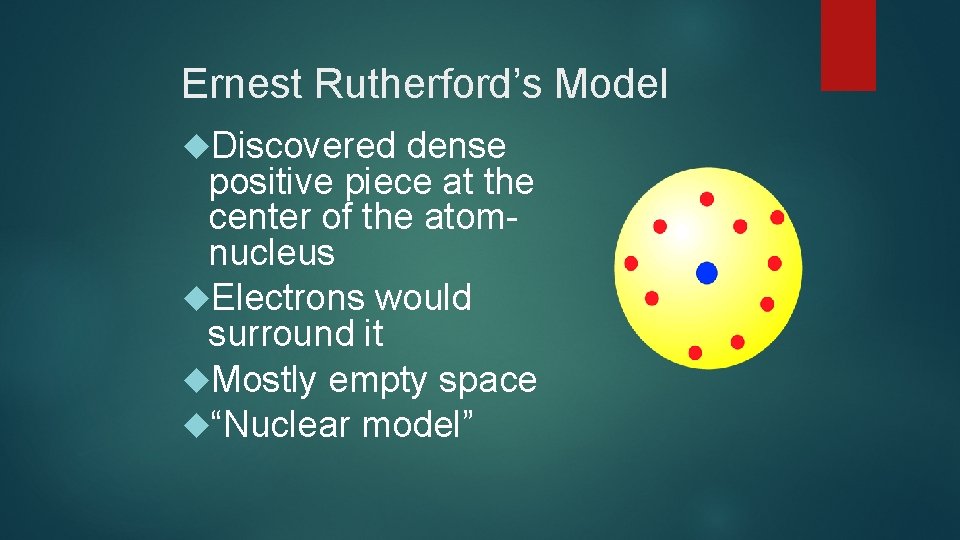
Ernest Rutherford’s Model Discovered dense positive piece at the center of the atomnucleus Electrons would surround it Mostly empty space “Nuclear model”
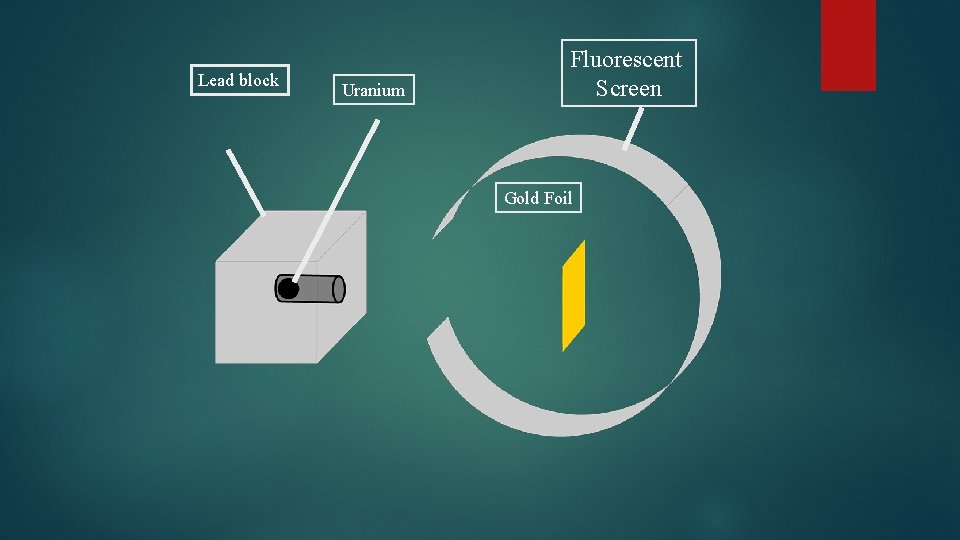
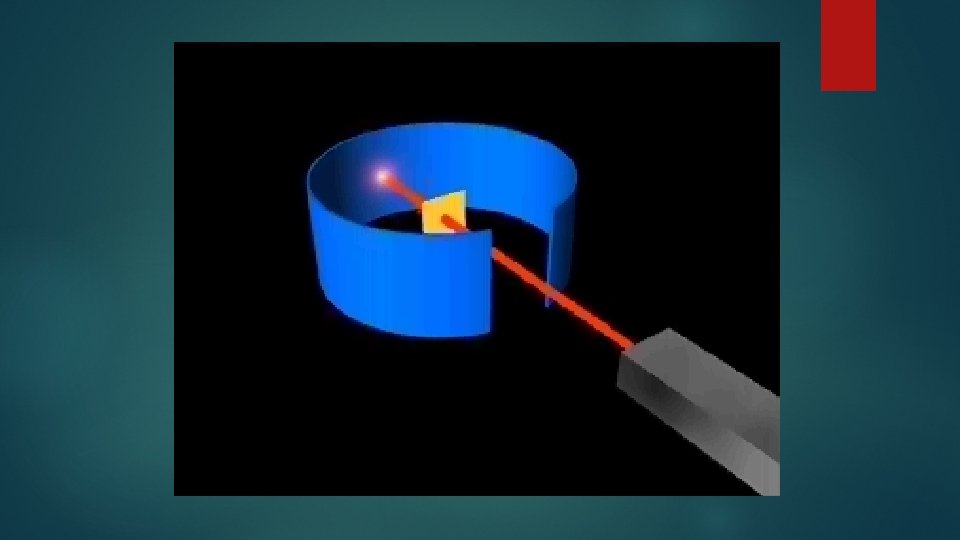
Lead block Uranium Fluorescent Screen Gold Foil
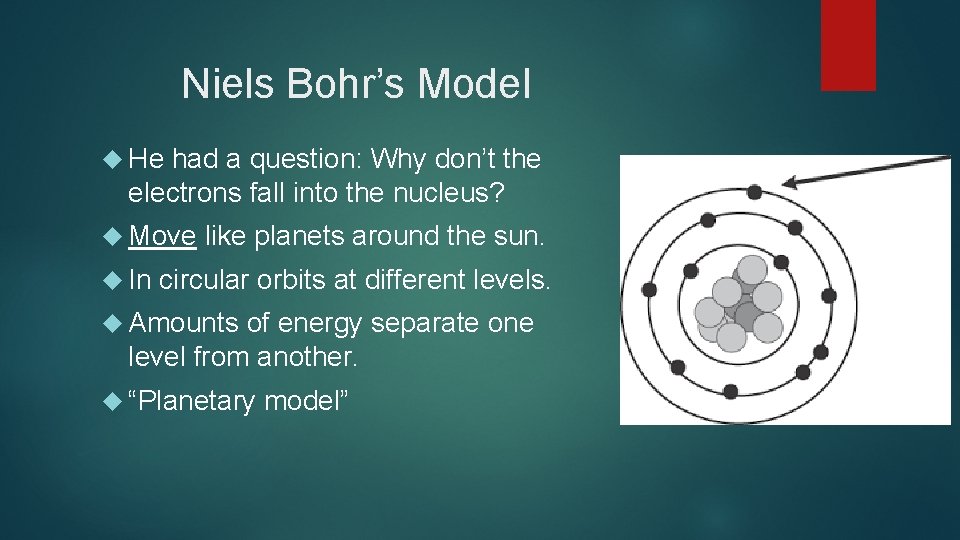
Niels Bohr’s Model He had a question: Why don’t the electrons fall into the nucleus? Move In like planets around the sun. circular orbits at different levels. Amounts of energy separate one level from another. “Planetary model”
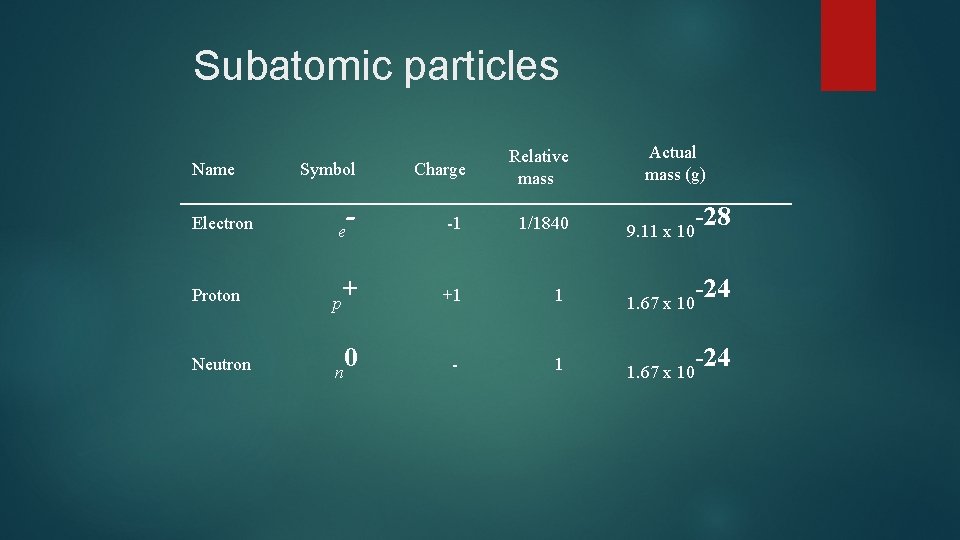
Subatomic particles Name Electron Actual mass (g) Symbol Charge Relative mass - -1 1/1840 9. 11 x 10 + +1 1 1. 67 x 10 0 - 1 1. 67 x 10 e Proton p Neutron n -28 -24
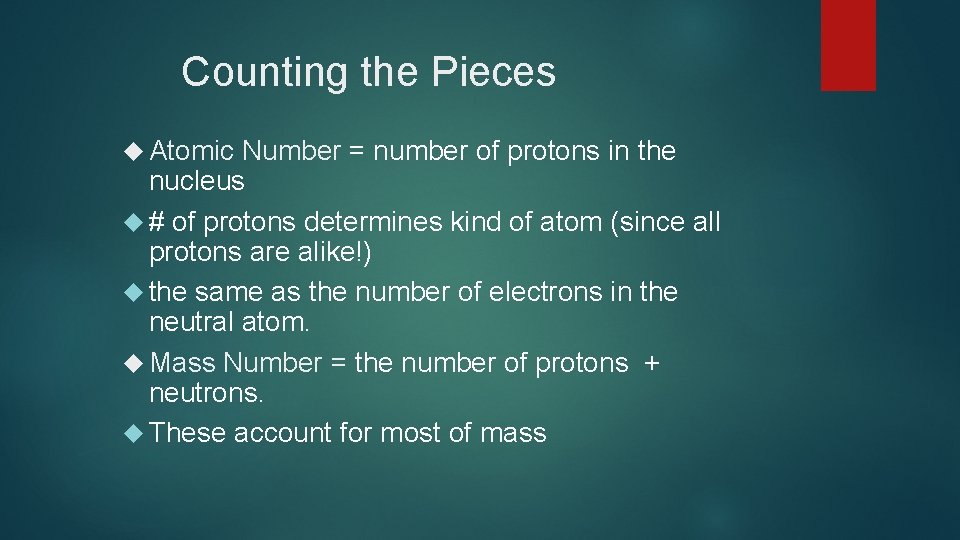
Counting the Pieces Atomic Number = number of protons in the nucleus # of protons determines kind of atom (since all protons are alike!) the same as the number of electrons in the neutral atom. Mass Number = the number of protons + neutrons. These account for most of mass
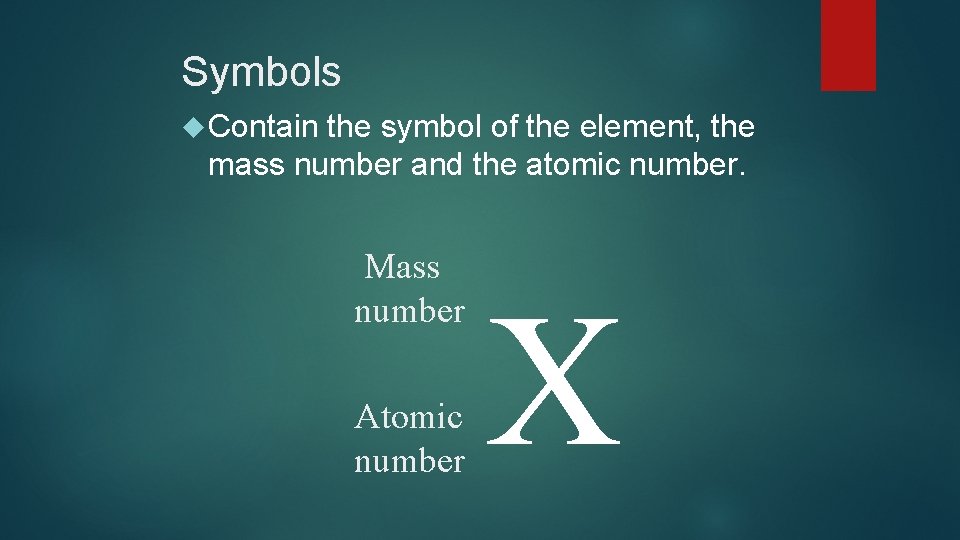
Symbols Contain the symbol of the element, the mass number and the atomic number. Mass number Atomic number X
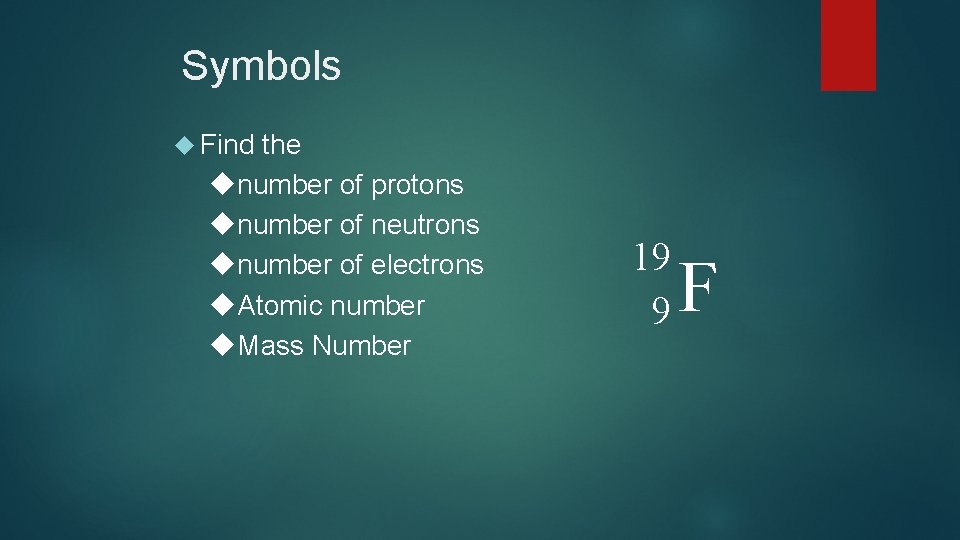
Symbols Find the number of protons number of neutrons number of electrons Atomic number Mass Number 19 9 F
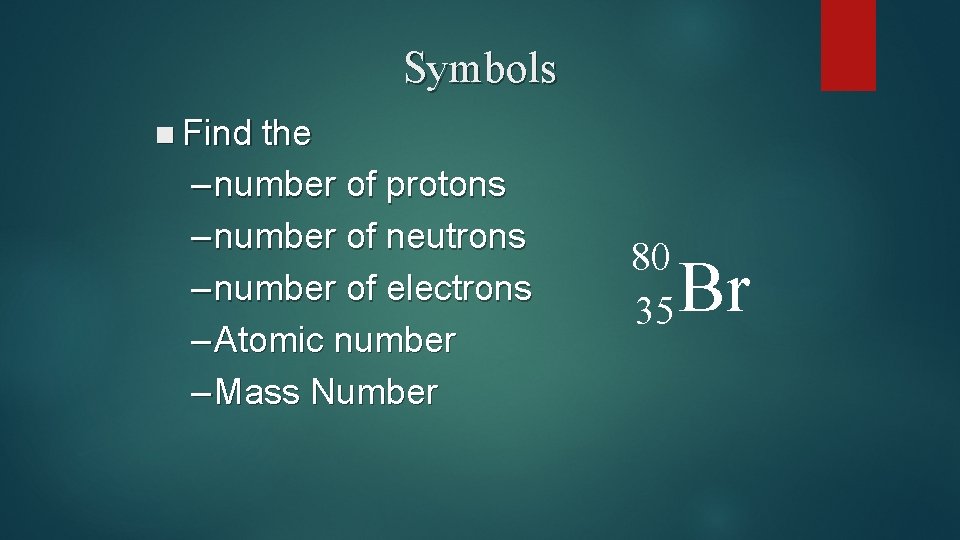
Symbols n Find the – number of protons – number of neutrons – number of electrons – Atomic number – Mass Number 80 35 Br
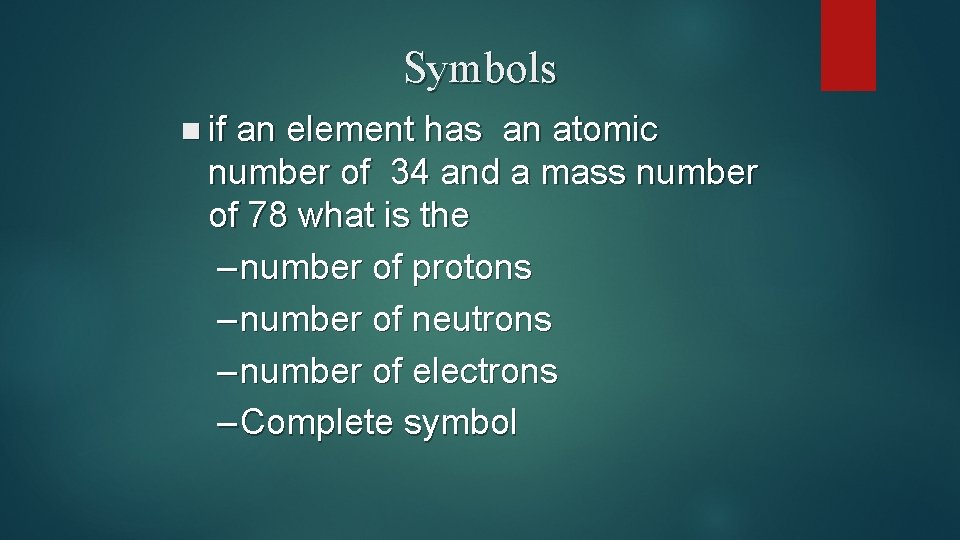
Symbols n if an element has an atomic number of 34 and a mass number of 78 what is the – number of protons – number of neutrons – number of electrons – Complete symbol
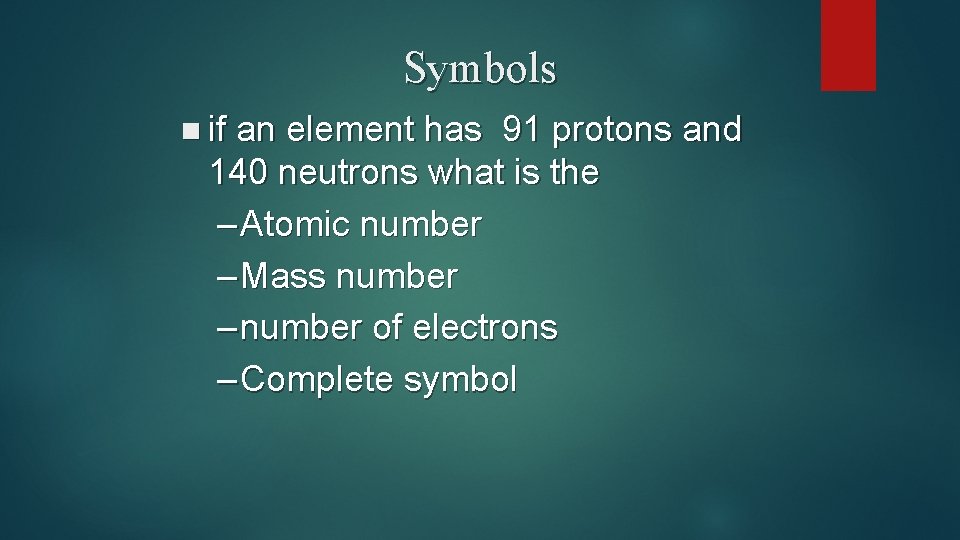
Symbols n if an element has 91 protons and 140 neutrons what is the – Atomic number – Mass number – number of electrons – Complete symbol
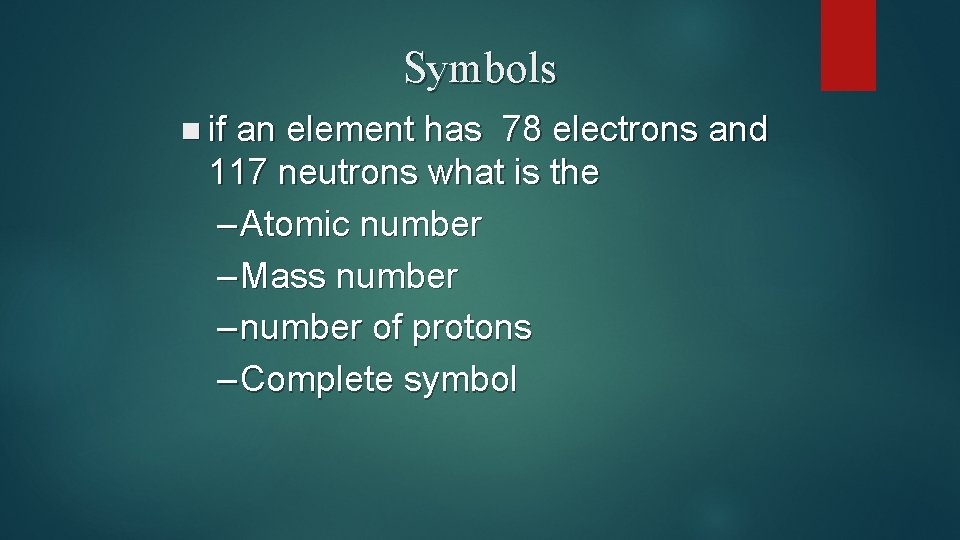
Symbols n if an element has 78 electrons and 117 neutrons what is the – Atomic number – Mass number – number of protons – Complete symbol

Isotopes Dalton was wrong. Atoms of the same element can have different numbers of neutrons. different mass numbers. called isotopes.

Naming Isotopes We can also put the mass number after the name of the element. carbon- 12 carbon -14 uranium-235 Uranium-238

Atomic Mass Is not a whole number because it is an average. are the decimal numbers on the periodic table.

Bohr Model

Light The study of light led to the development of the quantum mechanical model. Light is a kind of electromagnetic radiation. Electromagnetic radiation includes many kinds of waves All move at 3. 00 x 108 m/s = c
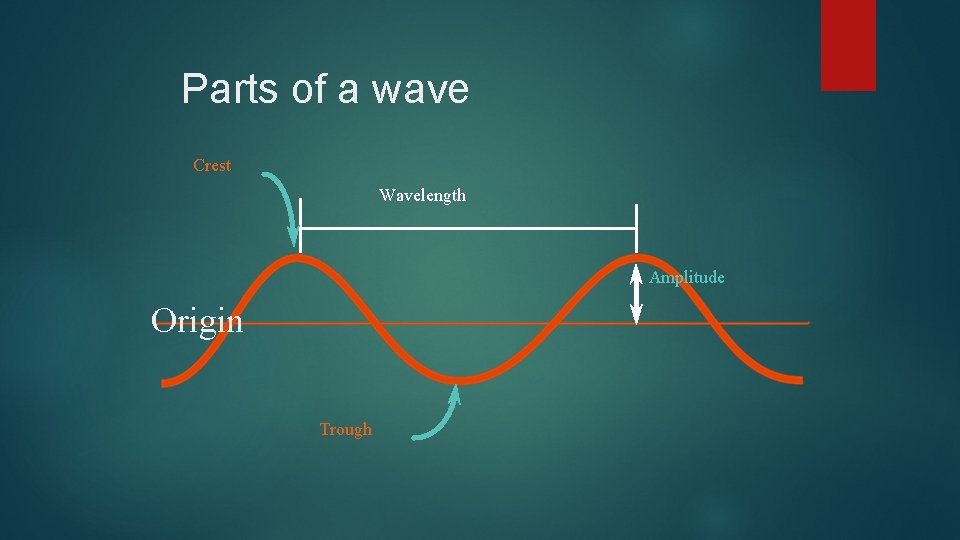
Parts of a wave Crest Wavelength Amplitude Origin Trough

Parts of Wave Origin - the base line of the energy. Crest - high point on a wave Trough - Low point on a wave Amplitude - distance from origin to crest Wavelength - distance from crest to crest Wavelength is abbreviated by the Greek letter lambda =

Frequency The number of waves that pass a given point per second. Units: cycles/sec or hertz (hz or sec-1) Abbreviated by Greek letter nu = n c =

Frequency and wavelength Are inversely related As one goes up the other goes down. Different frequencies of light are different colors of light. There is a wide variety of frequencies The whole range is called a spectrum, Fig. 13. 10, page 373
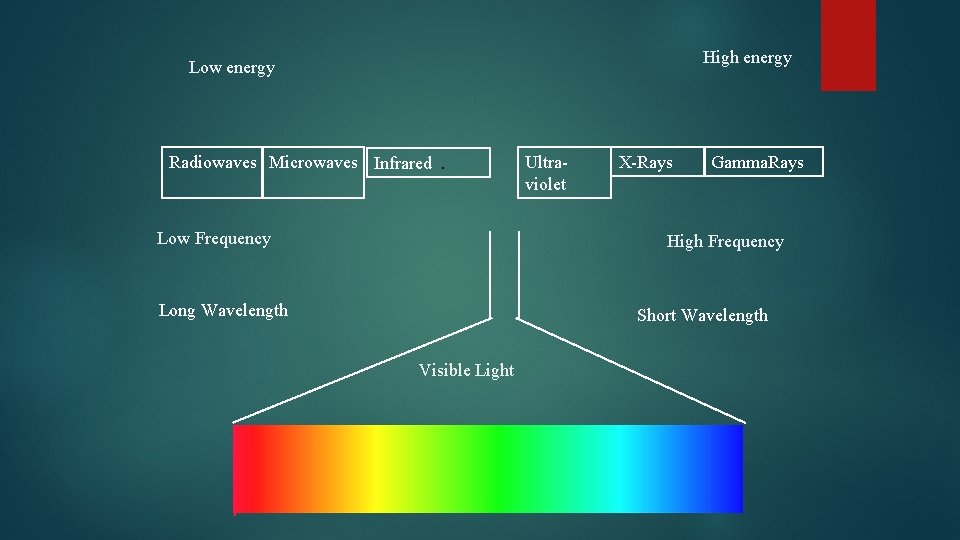
High energy Low energy Radiowaves Microwaves Infrared. Low Frequency Ultraviolet X-Rays Gamma. Rays High Frequency Long Wavelength Short Wavelength Visible Light

Prism White light is made up of all the colors of the visible spectrum. Passing it through a prism separates it.
If the light is not white By heating a gas with electricity we can get it to give off colors. Passing this light through a prism does something different.
Atomic Spectrum Each element gives off its own characteristic colors. Can be used to identify the atom. How we know what stars are made of.
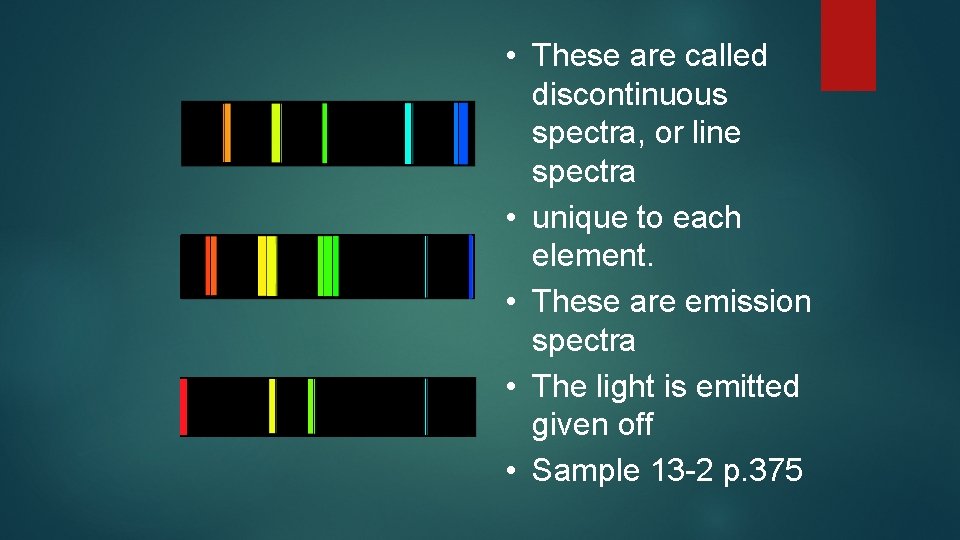
• These are called discontinuous spectra, or line spectra • unique to each element. • These are emission spectra • The light is emitted given off • Sample 13 -2 p. 375

Light is a Particle Energy is quantized. Light is energy Light must be quantized These smallest pieces of light are called photons. Photoelectric Energy effect? & frequency: directly related.

Energy and frequency =hx E is the energy of the photon is the frequency h is Planck’s constant h = 6. 6262 x 10 -34 Joules x sec. joule is the metric unit of Energy E
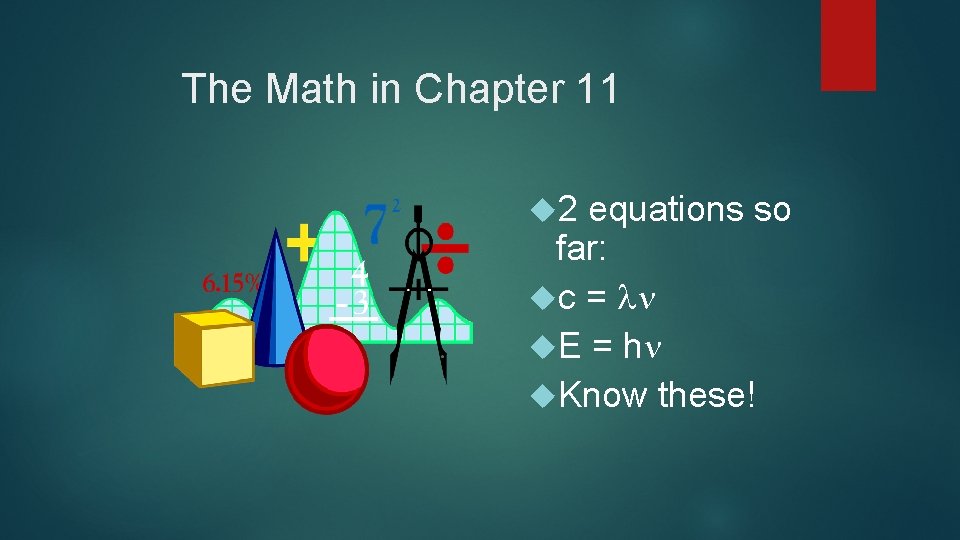
The Math in Chapter 11 2 equations so far: c = E = h Know these!

Examples What is the wavelength of blue light with a frequency of 8. 3 x 1015 hz? What is the frequency of red light with a wavelength of 4. 2 x 10 -5 m? What is the energy of a photon of each of the above?

Explanation of atomic spectra When we write electron configurations, we are writing the lowest energy. The energy level, and where the electron starts from, is called it’s ground state- the lowest energy level.
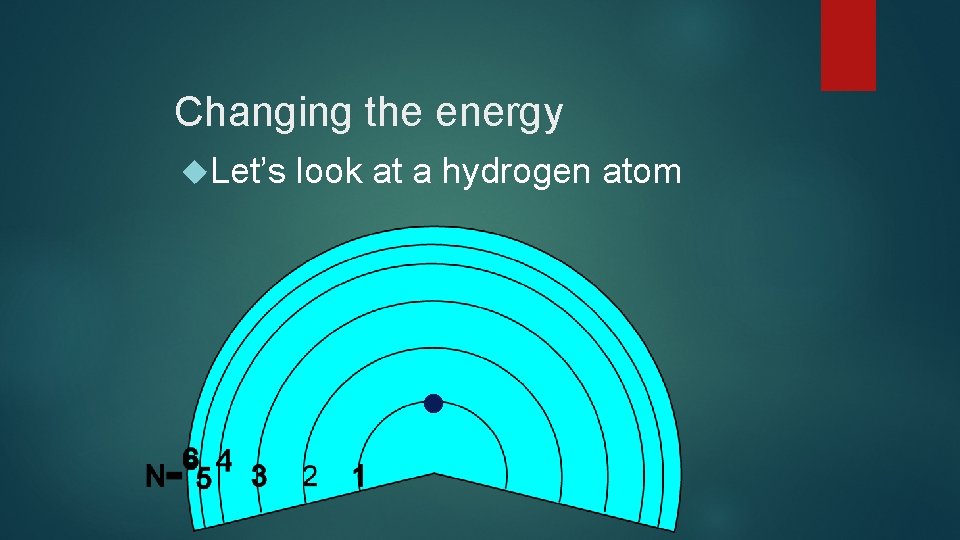
Changing the energy Let’s look at a hydrogen atom
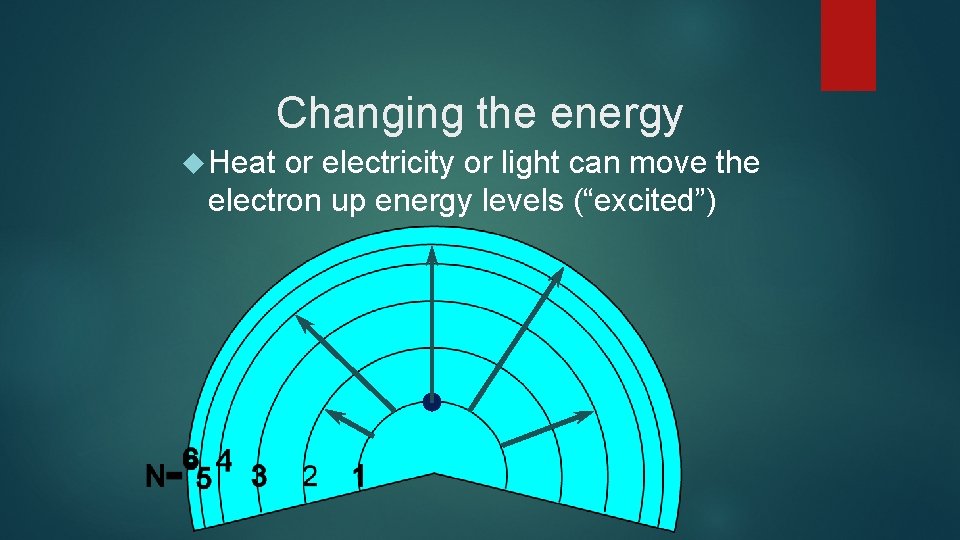
Changing the energy Heat or electricity or light can move the electron up energy levels (“excited”)
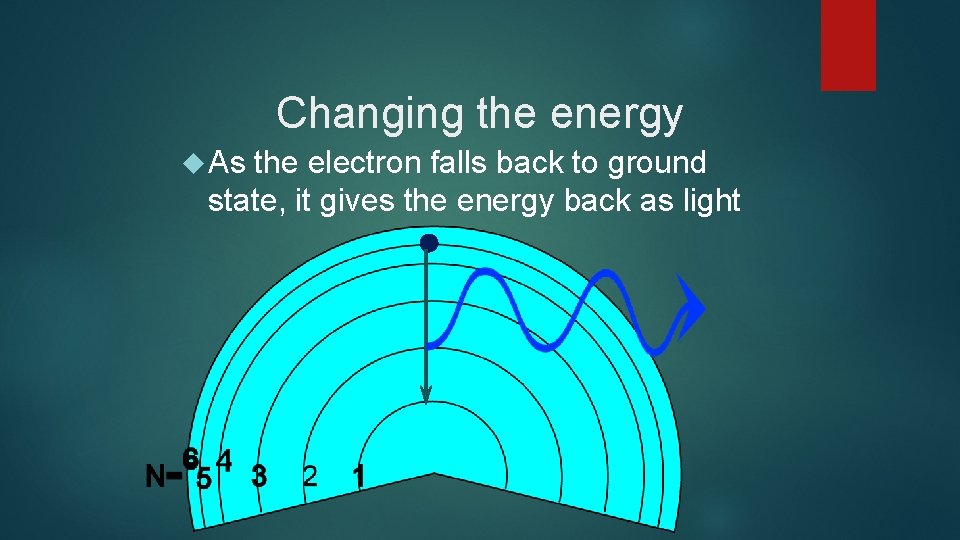
Changing the energy As the electron falls back to ground state, it gives the energy back as light

May Changing the energy fall down in steps Each with a different energy
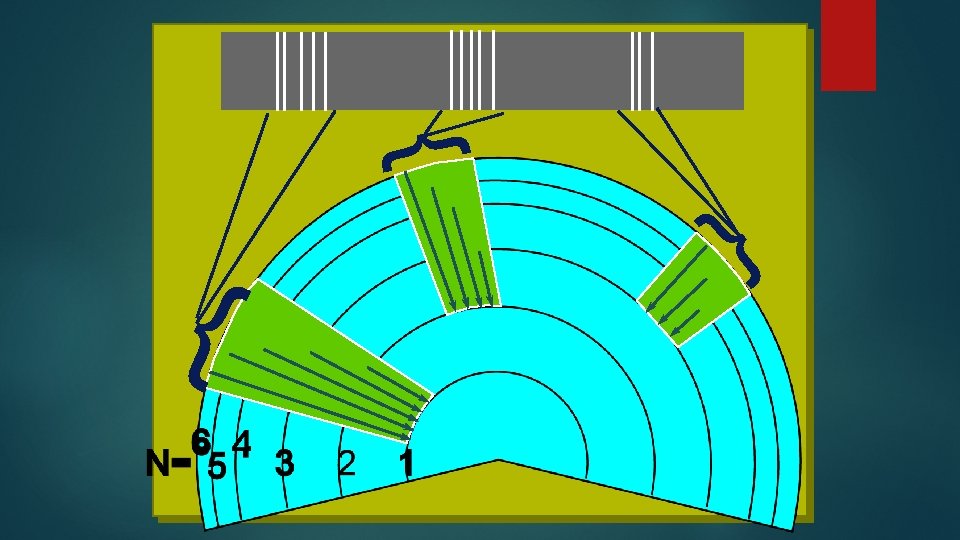

Ultraviolet Visible Infrared Further they fall, more energy, higher frequency. This is simplified the orbitals also have different energies inside energy levels All the electrons can move around.
- Slides: 42