Atomic Structure Introduction Atom indivisible in Greek Epicurus
Atomic Structure

Introduction • Atom = indivisible (in Greek) • Epicurus explained that matter can be broken down into very small invisible particles called atoms. • John Dalton proposed the atomic theory and explained the law of chemical combination. • Atom is the smallest possible amount of matter which still retains its identity as a chemical element.

• Atom is the smallest possible amount of matter which still retains its identity as a chemical element, consisting of a nucleus surrounded by electrons. • An atom is composed of a positively charged centre termed as “nucleus” and the central nucleus is surrounded by negatively charged electrons.
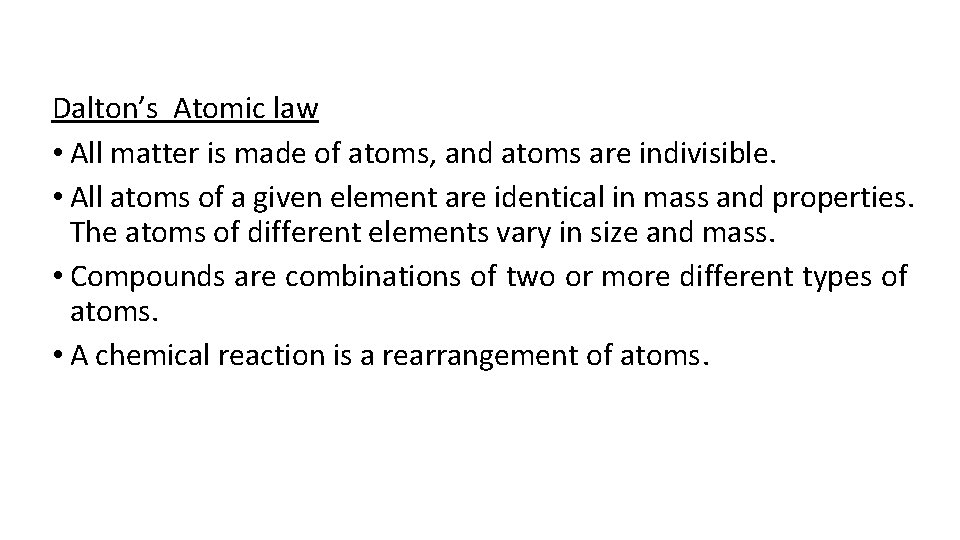
Dalton’s Atomic law • All matter is made of atoms, and atoms are indivisible. • All atoms of a given element are identical in mass and properties. The atoms of different elements vary in size and mass. • Compounds are combinations of two or more different types of atoms. • A chemical reaction is a rearrangement of atoms.

Dalton based his theory on; • the law of conservation of mass • the law of constant composition • The law of conservation of mass says that matter is not created or destroyed in a closed system. • The law of constant composition says that a pure compound will always have the same proportion of the same elements.
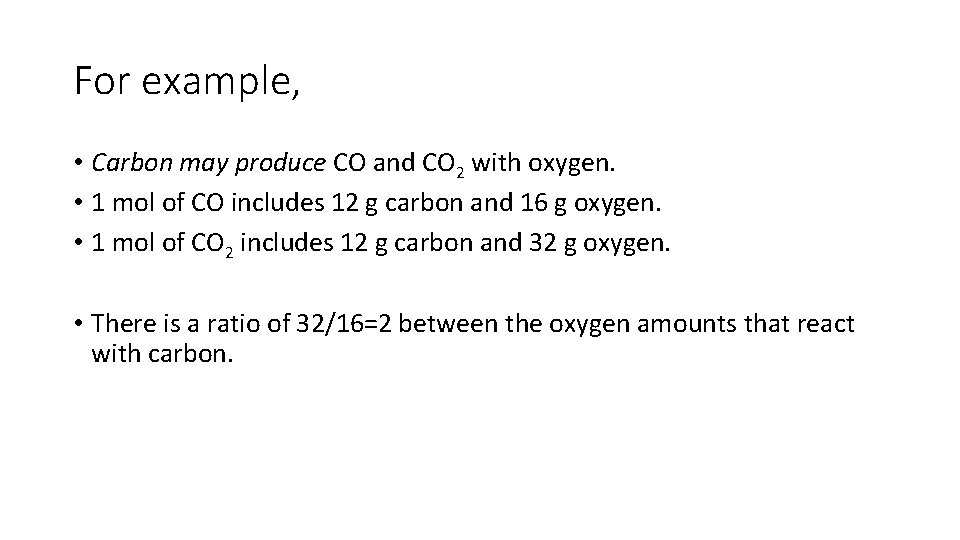
For example, • Carbon may produce CO and CO 2 with oxygen. • 1 mol of CO includes 12 g carbon and 16 g oxygen. • 1 mol of CO 2 includes 12 g carbon and 32 g oxygen. • There is a ratio of 32/16=2 between the oxygen amounts that react with carbon.

Electron • J. J Thompson proposed that an atom constitutes of at least one negatively charged particle called electron. • The charge of an electron is the negative charge of 1. 6× 1019 coulombs. • The relative mass of an electron is considered as 0.

Proton • Rutherford discovered protons with his gold foil experiment. • The absolute charge of a proton is the positive charge of 1. 6× 1019 coulomb. • The mass of a proton is 1. 6× 10 -24 g and is considered 1 that is mass of a hydrogen atom.

Neutron • Neutron was discovered by James Chadwick. • Neutron is represented by “n” and is considered a neutral particle. • The mass of a neutron is measured to be 1. 6 x 10 -24 g.

Millikan oil-drop experiment • Millikan determined the size of the charge on an electron in 1909 with an experiment called «oil-drop experiment» • Millikan created an electric field in a chamber and sprayed droplets of oil. Some oil drops became electrically charged and rise in spite of the gravity. • m. g = E. q • Since the mass (m), gravity (g), and (E) is known, the charge of an electron (q) can be calculated.

Atomic number • The number of protons in the nucleus of the atom is equal to the atomic number (Z). • Henry Moseley discovered the atomic number of each element using x-rays, which led to more accurate organization of the periodic table
- Slides: 11