Atomic Structure I Structure of the Atom Subatomic
Atomic Structure I. Structure of the Atom ¨ Subatomic Particles ¨ Electrons ¨ Protons ¨ Neutrons ¨ Atomic Mass
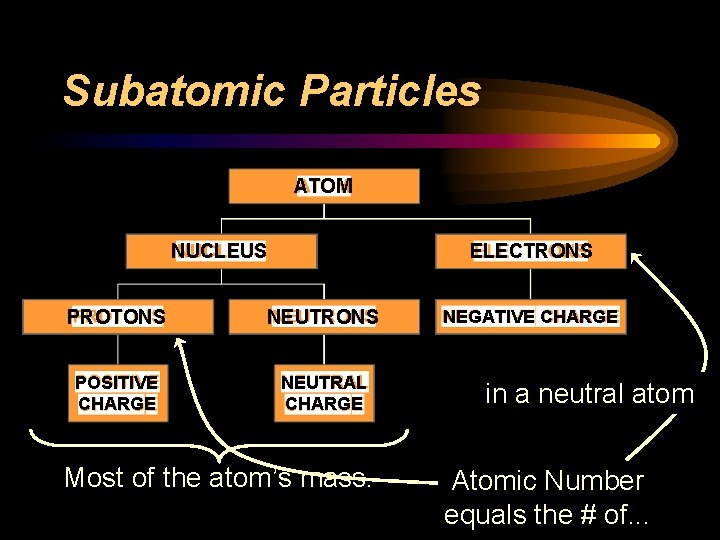
Subatomic Particles ATOM NUCLEUS ELECTRONS PROTONS NEUTRONS POSITIVE CHARGE NEUTRAL CHARGE Most of the atom’s mass. NEGATIVE CHARGE in a neutral atom Atomic Number equals the # of. . .
Subatomic Particles • The three main subatomic particles are distinguished by mass, charge, and location in the atom. • At the center of the atom is a small dense nucleus which is made of: • Protons • Neutrons
Subatomic Particles • Protons (+) • Particle with a positive charge • Located in the nucleus of an atom. • Neutrons (no charge) • Particle with no charge • Located in the nucleus of an atom.
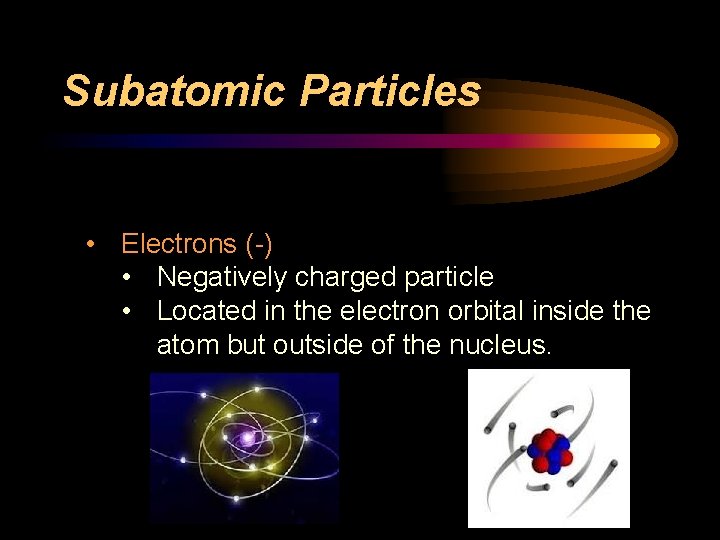
Subatomic Particles • Electrons (-) • Negatively charged particle • Located in the electron orbital inside the atom but outside of the nucleus.

Subatomic Particles • Distinguished by mass, charge, and location in the atom.
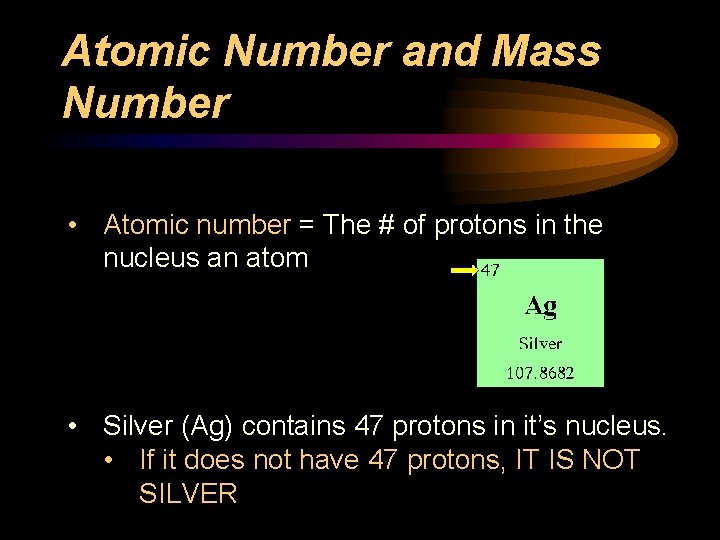
Atomic Number and Mass Number • Atomic number = The # of protons in the nucleus an atom • Silver (Ag) contains 47 protons in it’s nucleus. • If it does not have 47 protons, IT IS NOT SILVER
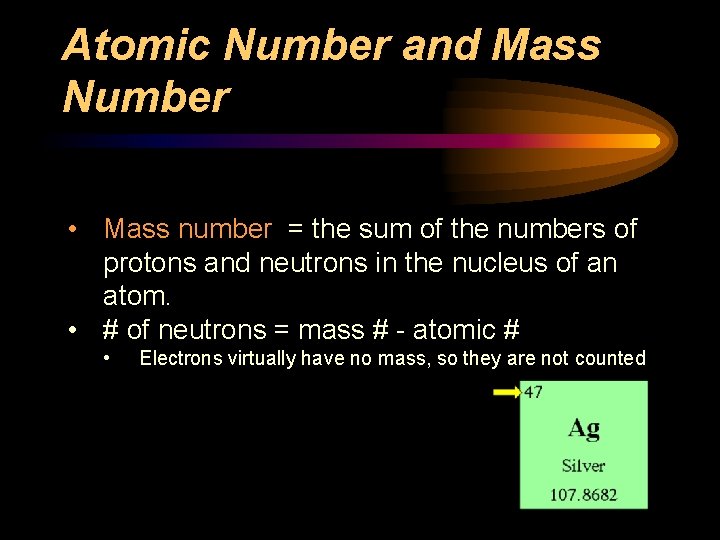
Atomic Number and Mass Number • Mass number = the sum of the numbers of protons and neutrons in the nucleus of an atom. • # of neutrons = mass # - atomic # • Electrons virtually have no mass, so they are not counted
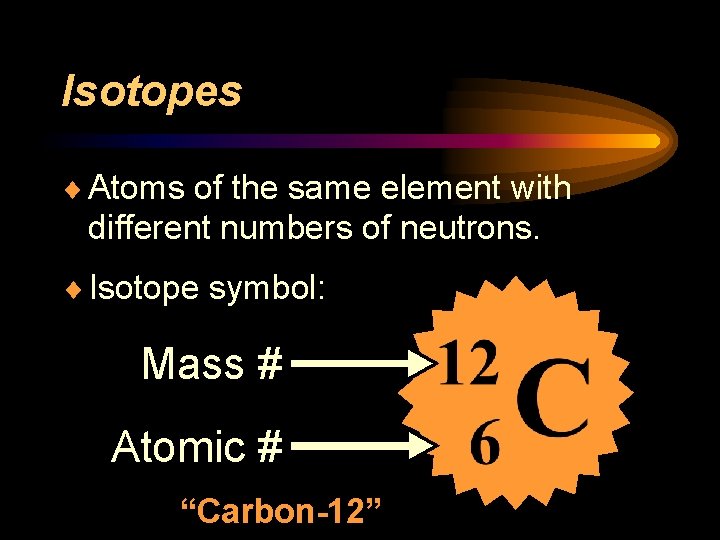
Isotopes ¨ Atoms of the same element with different numbers of neutrons. ¨ Isotope symbol: Mass # Atomic # “Carbon-12”
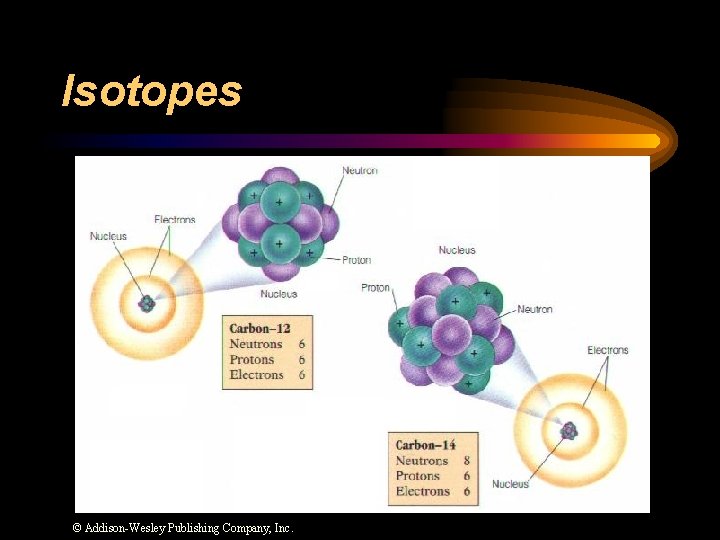
Isotopes © Addison-Wesley Publishing Company, Inc.
- Slides: 10