Atomic Structure History v 400 B C Democritus

Atomic Structure

History v 400 B. C. – Democritus proposed all matter was made up of small particles called atoms v Atomic numbers were assigned to elements by Henry Moseley v 1913 - Niels Bohr described the planetary model of the atom
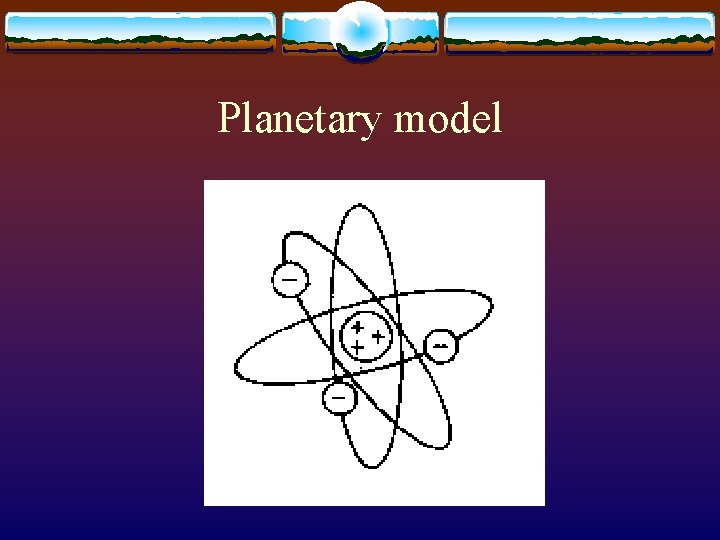
Planetary model

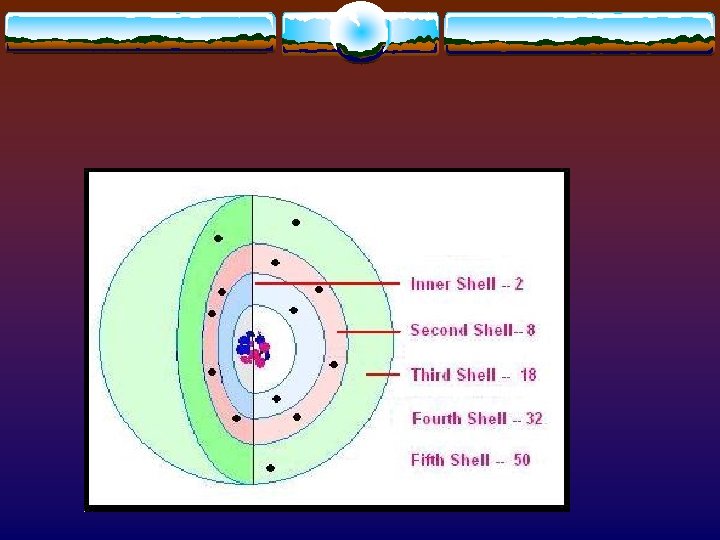
The Shell Model v The more accurate model puts a cloud around the nucleus and electrons move about in shells v Each shell can hold so many electrons v Little energy in the most inner shell so only 2 electrons can be found there. v The farther you get from the nucleus, the more electrons a shell can hold

The atoms makeup v This shell model of a hydrogen atom or lead atom is made up of the same general parts. v A nucleus made up of protons (1 in Hydrogen and 82 in Lead) and neutrons (usually the same amount as the protons) v Floating around the nucleus are electrons

Mass of Subatomic Particles Particle Charge Mass compared Actual Mass to electron Electron Proton Neutron Negative 1 9. 11 x 10 -31 Positive 1836 1. 673 x 10 -27 Neutral 1841 1. 675 x 10 -27

Charges v The three subatomic particles as they are called have different charges v Protons- (+) v Neutrons- no charge v Electrons- (-)

v

Atomic Mass v The mass of an atom is equal to the number of protons and neutrons found in the nucleus v If an atom of carbon has 6 protons and 6 neutrons, its mass is 12

Atomic Number v The atomic number found above the chemical symbol is equal to the number of protons the atom has v An atom with 19 protons has to be potassium (K)

Chemical Names v Why is Co different from CO? v Co is Cobalt v CO is carbon monoxide v Be sure to use upper and lower case properly

Isotopes v Atoms of the same element that have different numbers of neutrons are called isotopes v Hydrogen can have 0, 1, or 2 neutrons in it’s nucleus

Misc. Facts v Hydrogen is the most abundant element. It makes up almost 90% of all atoms. As well it was the 1 st element to exist v Fused hydrogen atoms formed inside stars and this created the other elements v Most atoms formed when the universe began and are constantly being recycled v Atoms that make up ‘you’ are billions of years old

Names w/ 1 letter that begins name Names w/ 2 letters and both letters are start of name Carbon Neon Names w/ 2 letters and 1 st letter starts and second is heard later in name Magnesium Symbol has nothing to do with name Sodium

Chapter Review (class work) v Try to complete #5, 6, and 7.

Protons have a positive charge A. B. True False

C-12 is a radioactive isotope used in carbon dating fossils A. B. True False

The charge of a neutron is A. B. negative No charge

The number of electrons that can fit in the 1 st shell is A. B. C. 8 2 18

The electron has more mass than the proton and neutron A. B. Yes No

The atomic number of carbon is A. B. C. D. 12. 01115 12 6 C

The element symbol of helium is A. B. C. D. H 2 He Helium

The mass number of C-14 is 14 A. B. True False

Play-doh Lab Partner up v Create a 3 dimensional model of an atom using play-doh and beads. It must be the shell model v Your atom must have an atomic number between 6 and 12. v On a separate sheet draw your atom and label the following: Protons, neutrons, electrons, nucleus, electron cloud, any and all shells v Write down the name, atomic number, atomic mass, and symbol v
- Slides: 25