Atomic Structure History of Atomic Theory Democritus 460















- Slides: 25

Atomic Structure History of Atomic Theory

Democritus (460 - 370 BC) Idea of indivisible particles - atomos

Aristotle (460 - 370 BC) Dismissed Democritus’ idea Earth/Wind/Water/Fire Fast forward 2000 years….

John Dalton (1766 -1844) • No actual experiments • Brought idea of an indivisible particle back into fashion

John Dalton (1766 -1844) • His model:

Dalton’s Atomic Laws 1. Law of Conservation of Mass 2. Law of Definite Proportions

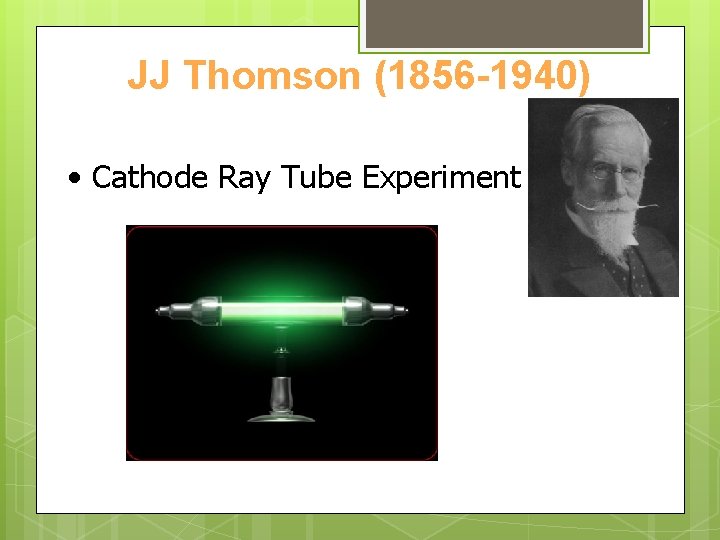
JJ Thomson (1856 -1940) • Cathode Ray Tube Experiment

Cathode Ray Tube Experiment Video Clip https: //www. youtube. com/watc h? v=Csj. LYLW_3 G 0

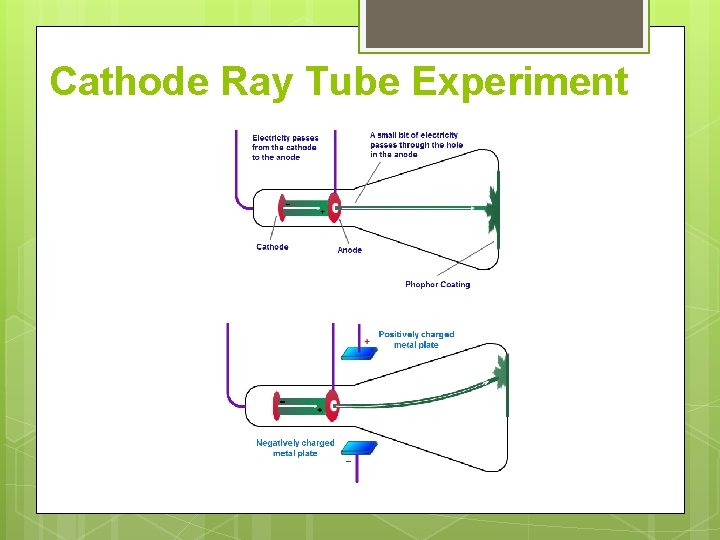
Cathode Ray Tube Experiment

Cathode Ray Tube Experiment • Rays must have been made up of very small, light, negatively charged particles: • Electrons.

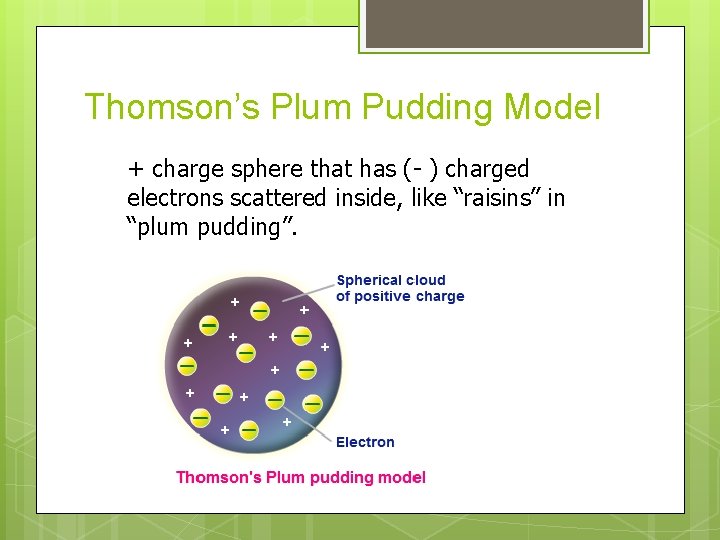
Thomson’s Plum Pudding Model + charge sphere that has (- ) charged electrons scattered inside, like “raisins” in “plum pudding”.

Ernest Rutherford (1871 -1937) Alpha particles (+ charged) newly discovered Theorized they should pass through the positive “dough” of the atom

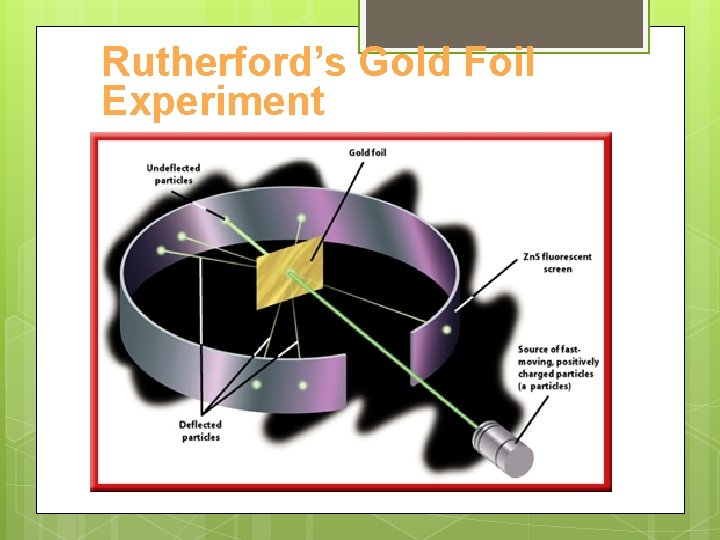
Rutherford’s Gold Foil Experiment https: //www. youtube. com/w atch? v=ecsg. C 1 w. Sp 5 I

Rutherford’s Gold Foil Experiment

Rutherford’s Gold Foil Experiment • Most particles passed through as expected • 1% deflected back • Conclusions: • Atoms must be mostly empty space • Must be a small, dense area of positive charge in the center of the atom: The Nucleus

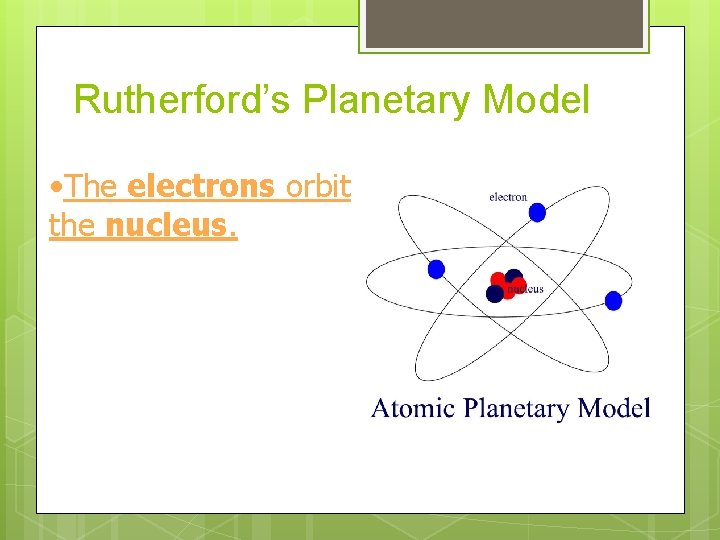
Rutherford’s Planetary Model • The electrons orbit the nucleus.

Niels Bohr (1885 -1962) Worked in Rutherford’s lab Wondered why electrons are not attracted to the + nucleus and cluster around it

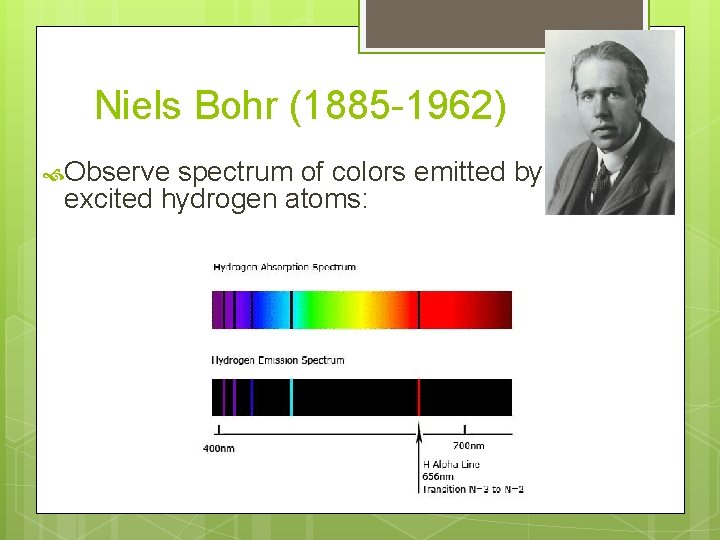
Niels Bohr (1885 -1962) Observe spectrum of colors emitted by excited hydrogen atoms:

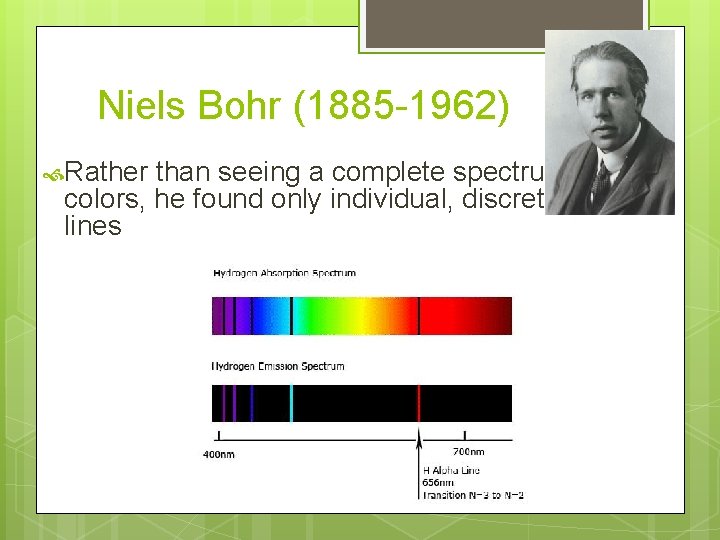
Niels Bohr (1885 -1962) Rather than seeing a complete spectrum of colors, he found only individual, discrete lines

Niels Bohr (1885 -1962) Bohr hypothesized that atoms had quantized energy levels

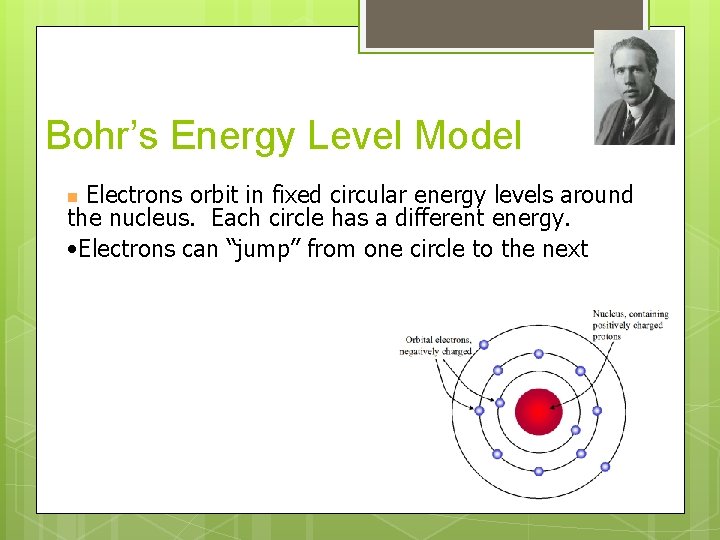
Bohr’s Energy Level Model Electrons orbit in fixed circular energy levels around the nucleus. Each circle has a different energy. • Electrons can “jump” from one circle to the next n

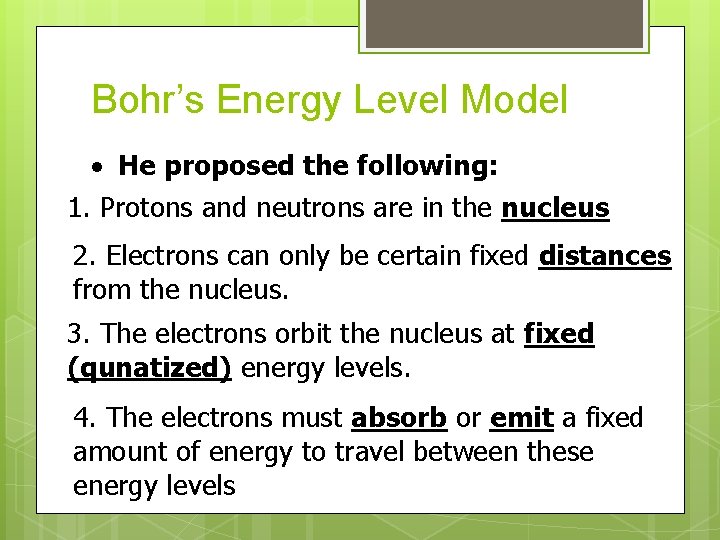
Bohr’s Energy Level Model · He proposed the following: 1. Protons and neutrons are in the nucleus 2. Electrons can only be certain fixed distances from the nucleus. 3. The electrons orbit the nucleus at fixed (qunatized) energy levels. 4. The electrons must absorb or emit a fixed amount of energy to travel between these energy levels

Review Who is the father of atomic theory? Dalton What was the first model of the atom? Dalton’s Tiny Ball Model What are Dalton’s 3 Laws? Law of Conservation of Mass, Law of Constant Composition, Law of Multiple Porportion

Review How were Thomson’s and Dalton’s model different? Dalton’s model was 1 sphere that cannot be divided, Thomson had the plum pudding where electrons are randomly spread throughout a positively charged sphere. What did Thomson find out? Atoms have electrons, they have a - charge

Review What were Rutherford’s conclusions from the Gold Foil Experiment? Atom has a positively charged nucleus electrons are outside the nucleus atoms are mostly empty Nucleus contains most of the mass.