Atomic Structure Electron Configuration Valence Electrons and Ions





- Slides: 10

Atomic Structure Electron Configuration, Valence Electrons, and Ions

Quick Review • Energy shells hold electrons around nucleus – 1 st Shell can hold 2 electrons – 2 nd Shell can hold 8 electrons – 3 rd Shell can hold 18 electrons* _ _ • *We only ever put 8 electrons in the 3 rd _ _ _ Nucleus _ _ _ _ _

What Atoms Want to Do • VALENCE ELECTRONS: electrons in the outermost energy shell • Atoms want their outermost shell filled with as many valence electrons it can hold: How many valence electrons? 3 protons (3+) 4 neutrons (0) 2 ____ – 2 valence e- in the 1 st shell – 8 valence e- in the 2 nd shell – 8 valence e- in the 3 rd shell • Atoms will take, give, or share electrons to have the outermost shell filled How many valence electrons? 6 ____ 8 protons (8+) 8 neutrons (0)

Valence Electrons Rules • Rules to determine if an atom will gain or lose valence electrons 1. If atom has 8 valence electrons. . . • • • No gain or loss Is neutral (this includes helium with 2) 2. If atom has 1 -3 valence electrons… • • Loses them Becomes a positive (+) ion= Cation 3. If atom has 5 -7 valence electrons… • • Gains however many it needs to have 8 Becomes a negative (-) ion = Anion 4. Don’t worry about atoms with exactly 4 valence electrons

Making Ions • Atoms will either gain or lose valence electrons to make sure the outermost shell is full – Losing or gaining valence electrons makes the atom an ion and gives it a (+) or (–) charge • ION: A positively or negatively charged atom (the atom has lost or gained an electron • 2 Ways to Determine the Charge. . . 1. Protons – Electrons • • 11 protons – 10 electrons = 1+ charge 15 protons – 18 electrons = 3 - charge 2. # of valence electrons lost or gained • • Lost 2 valence electrons = 2+ charge Gained 1 valence electron = 1 - charge

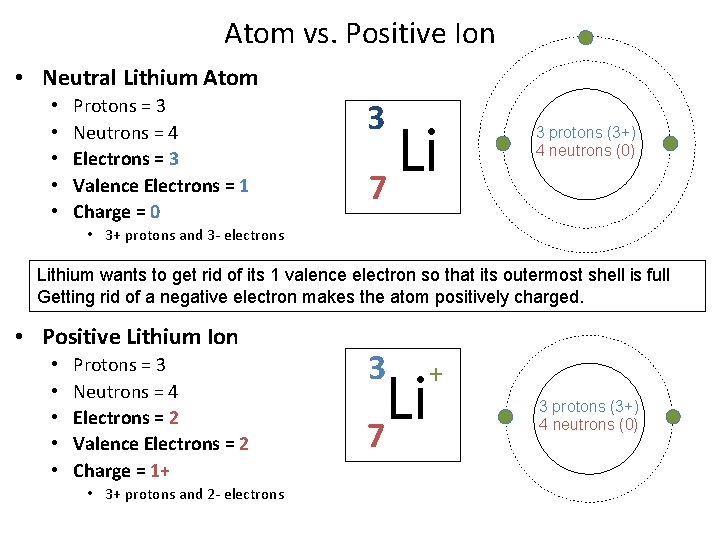
Atom vs. Positive Ion • Neutral Lithium Atom • • • Protons = 3 Neutrons = 4 Electrons = 3 Valence Electrons = 1 Charge = 0 3 Li 7 3 protons (3+) 4 neutrons (0) • 3+ protons and 3 - electrons Lithium wants to get rid of its 1 valence electron so that its outermost shell is full Getting rid of a negative electron makes the atom positively charged. • Positive Lithium Ion • • • Protons = 3 Neutrons = 4 Electrons = 2 Valence Electrons = 2 Charge = 1+ • 3+ protons and 2 - electrons 3 Li 7 + 3 protons (3+) 4 neutrons (0)

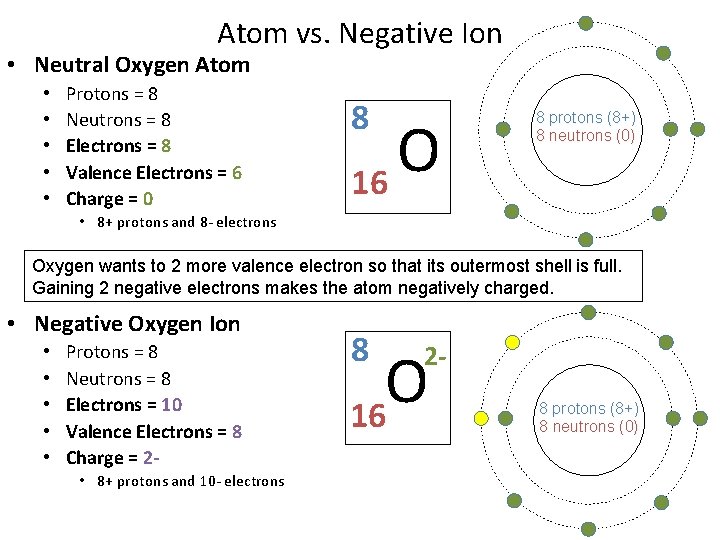
Atom vs. Negative Ion • Neutral Oxygen Atom • • • Protons = 8 Neutrons = 8 Electrons = 8 Valence Electrons = 6 Charge = 0 8 O 16 8 protons (8+) 8 neutrons (0) • 8+ protons and 8 - electrons Oxygen wants to 2 more valence electron so that its outermost shell is full. Gaining 2 negative electrons makes the atom negatively charged. • Negative Oxygen Ion • • • Protons = 8 Neutrons = 8 Electrons = 10 Valence Electrons = 8 Charge = 2 • 8+ protons and 10 - electrons 8 2 - O 16 8 protons (8+) 8 neutrons (0)

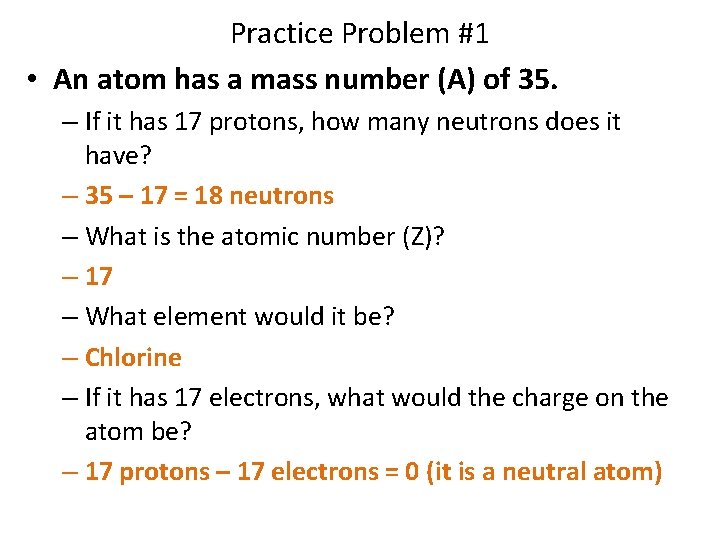
Practice Problem #1 • An atom has a mass number (A) of 35. – If it has 17 protons, how many neutrons does it have? – 35 – 17 = 18 neutrons – What is the atomic number (Z)? – 17 – What element would it be? – Chlorine – If it has 17 electrons, what would the charge on the atom be? – 17 protons – 17 electrons = 0 (it is a neutral atom)

Practice Problem #2 • An atom has a mass number (A) of 24. – If it has 12 protons, how many neutrons does it have? – 24 – 12 = 12 neutrons – What is the atomic number (Z)? – 12 – What element would it be? – Magnesium – If it has 10 electrons, what would the charge on the atom be? – 12 protons – 10 electrons = 2+ (it is an ion)
Practice Problem #3 • An atom has a mass number (A) of 14. – If it has 7 protons, how many neutrons does it have? – 14 – 7 = 7 neutrons – What is the atomic number (Z) – 7 – What element would it be? – Nitrogen – If it has 10 electrons, what would the charge on the atom be? – 7 protons – 10 electrons = 3 - (it is an ion)