Atomic Structure Electricity and Charging Basic Subatomic Particles

Atomic Structure, Electricity and Charging
Basic Subatomic Particles Neutron Proton Electron

Charges Two types Positive (protons) Negative (electrons) No Charge = Neutral

Attracting and Repelling Like charges repel Opposite charges attract

Nucleus Composed of Neutrons and Protons held together by the Strong Nuclear Force

Fundamental Forces Strong Nuclear Electromagnetic Weak Nuclear Gravity 38 10 36 10 13 10 1

Electricity Energy due to the orientation or movement of charges
Which Charge Moves? Electricity depends on the movement of electrons Protons DO NOT move!!!

Conductors Allow electrons to easily move through material
Insulators Restrict the movement of electrons

Electrostatic Charging l. Friction l. Contact l. Induction
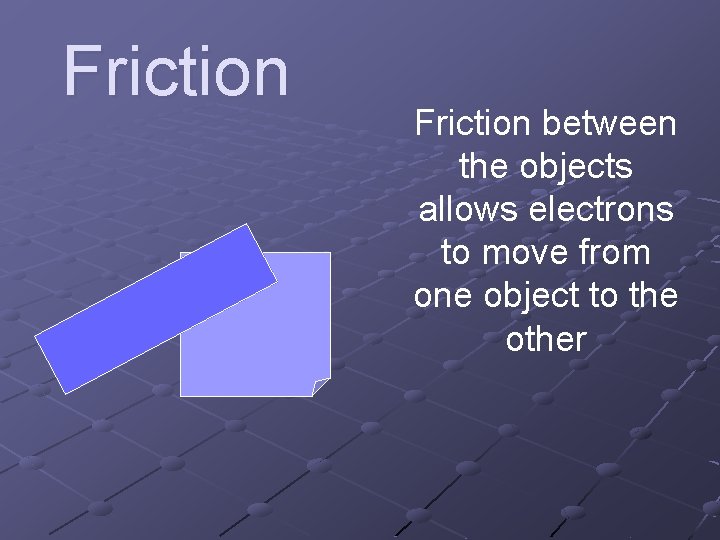
Friction between the objects allows electrons to move from one object to the other
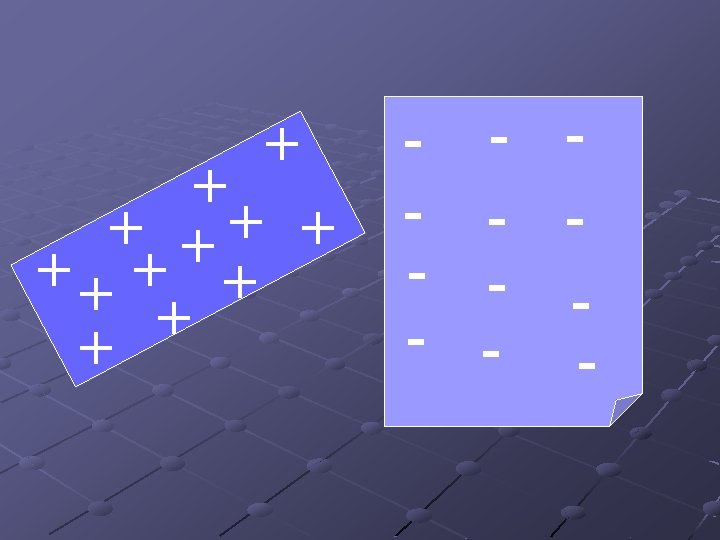
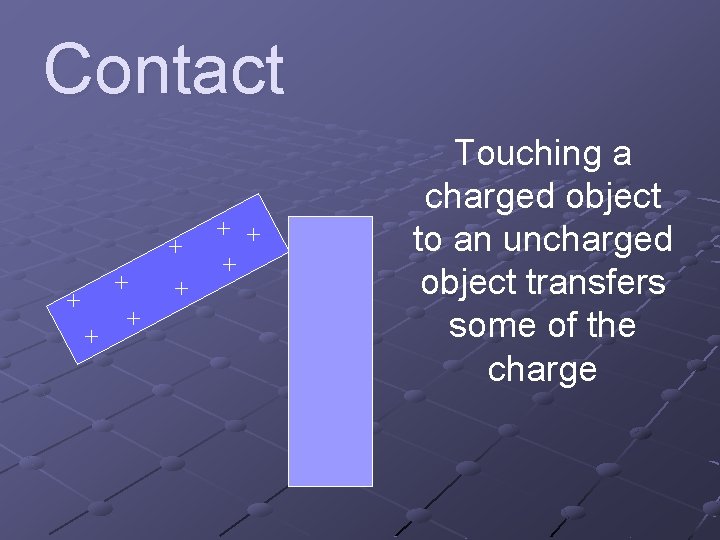
Contact + + + + + Touching a charged object to an uncharged object transfers some of the charge
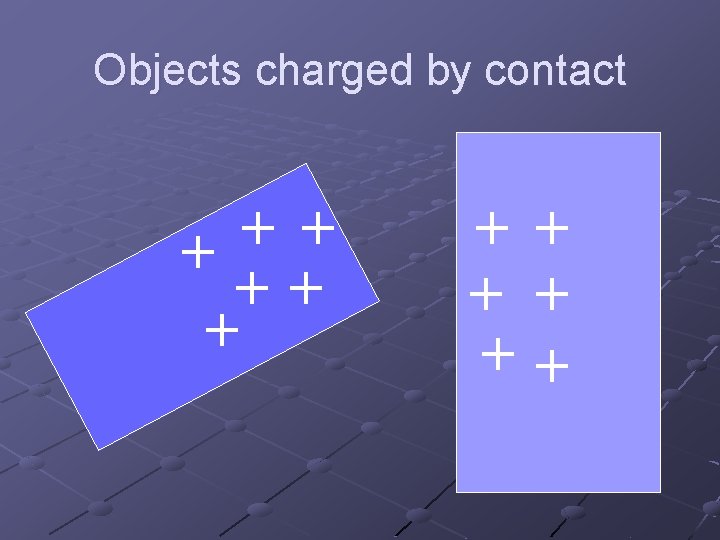
Objects charged by contact + + + ++ + +
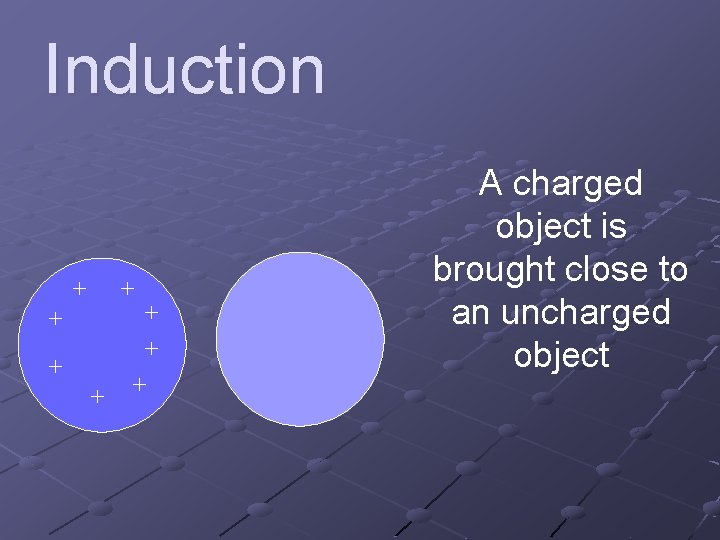
Induction + + + + A charged object is brought close to an uncharged object
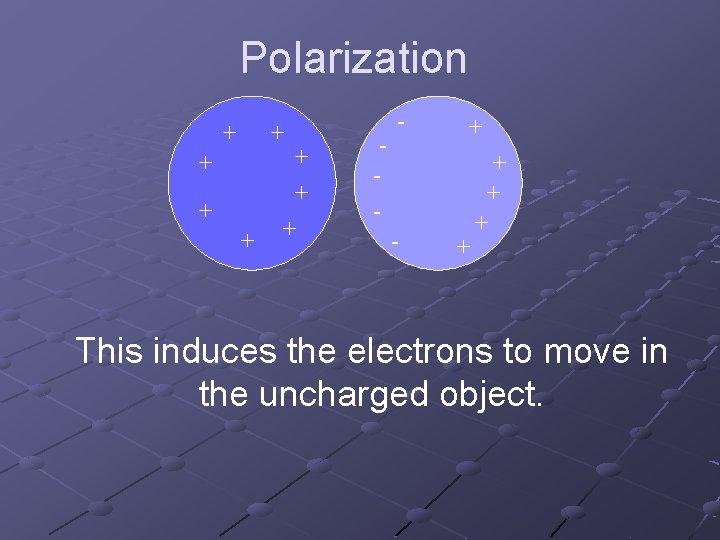
Polarization + + + + - - - + + + This induces the electrons to move in the uncharged object.
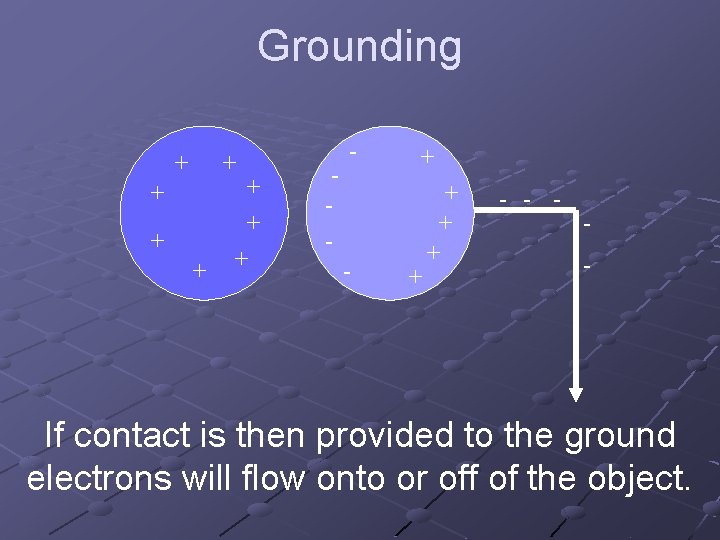
Grounding + + + + - - - + + + - - If contact is then provided to the ground electrons will flow onto or off of the object.
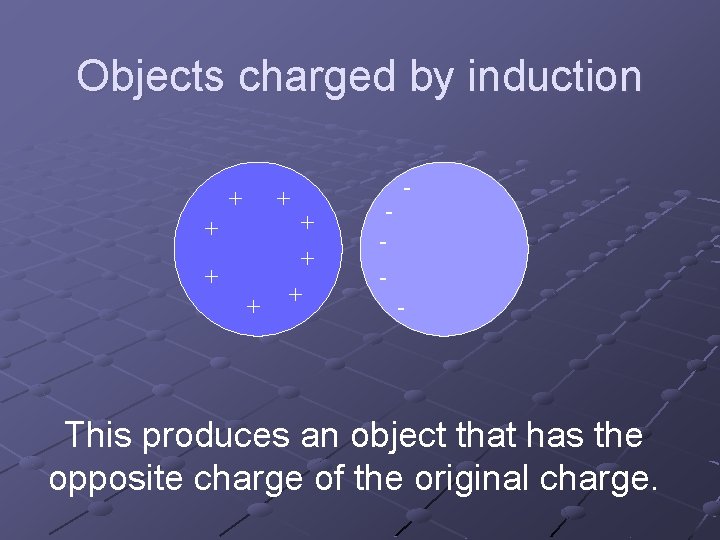
Objects charged by induction + + + + - - - This produces an object that has the opposite charge of the original charge.

Conservation of Charges are neither created nor destroyed but are moved from place to place and object to object.
- Slides: 20