Atomic Structure Discovery of the Electron What is







- Slides: 30

Atomic Structure Discovery of the Electron

What is the relationship between electrical charge and the structure of atoms? • Historical Background – • Early 1800’s matter is composed of atoms • 19 th century – what is the nature of electricity and magnetism?

What is the relationship between electrical charge and the structure of atoms? • Historical Background – • Early 1800’s matter is composed of atoms • 19 th century – what is the nature of electricity and magnetism?

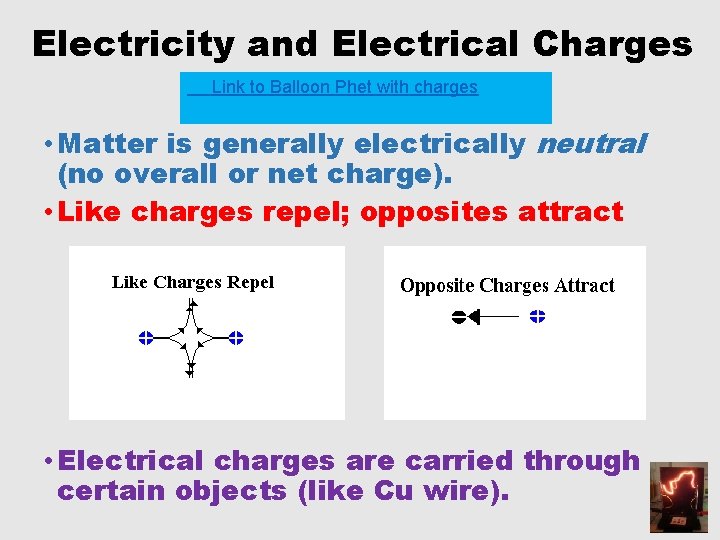
Electricity and Electrical Charges Link to Balloon Phet with charges • Matter is generally electrically neutral (no overall or net charge). • Like charges repel; opposites attract • Electrical charges are carried through certain objects (like Cu wire).

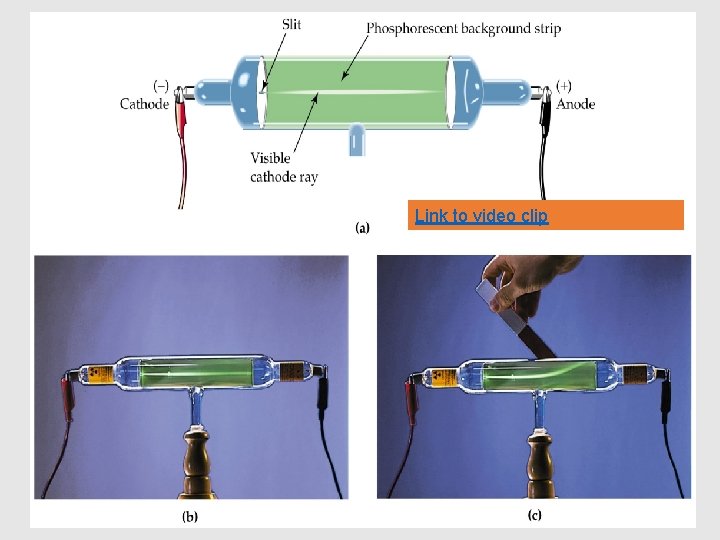
The Mysterious Cathode Ray Link to intro clip • What could it be made of ? • Light? • Stream of Particles?

Link to video clip

What does it tell us if the cathode ray is deflected by a magnet? • Is it made of ordinary light or particles? Ordinary light is not deflected by a magnet. Electrically charged particles are attracted to a magnet.

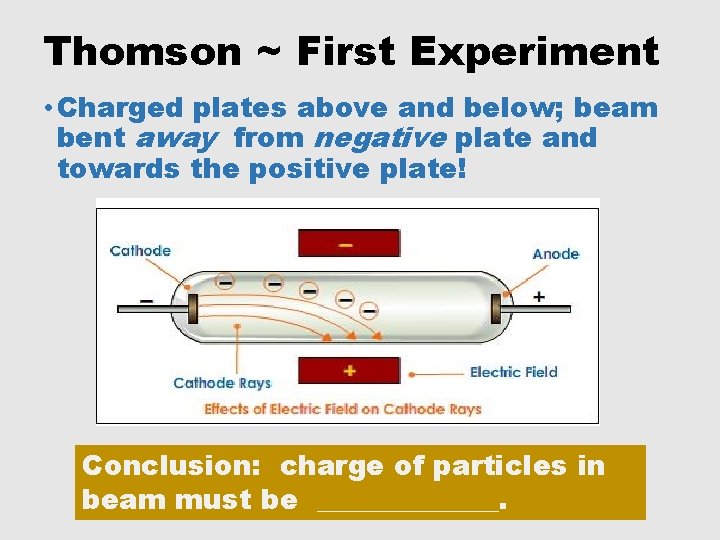
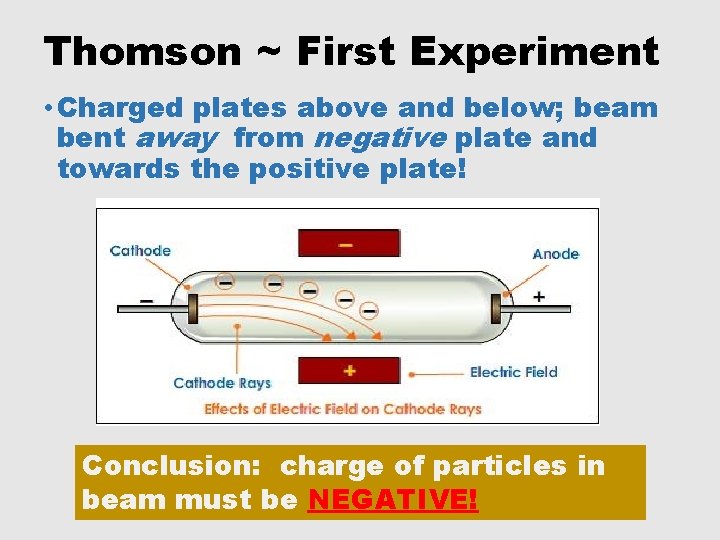
Thomson ~ First Experiment • Charged plates above and below; beam bent away from negative plate and towards the positive plate! Conclusion: charge of particles in beam must be _______.

Thomson ~ First Experiment • Charged plates above and below; beam bent away from negative plate and towards the positive plate! Conclusion: charge of particles in beam must be NEGATIVE!

What do we know about cathode rays so far? • Particle, not ordinary light • Negatively Charged • What is a property that we could measure that could help identify a particle that is too small to see?

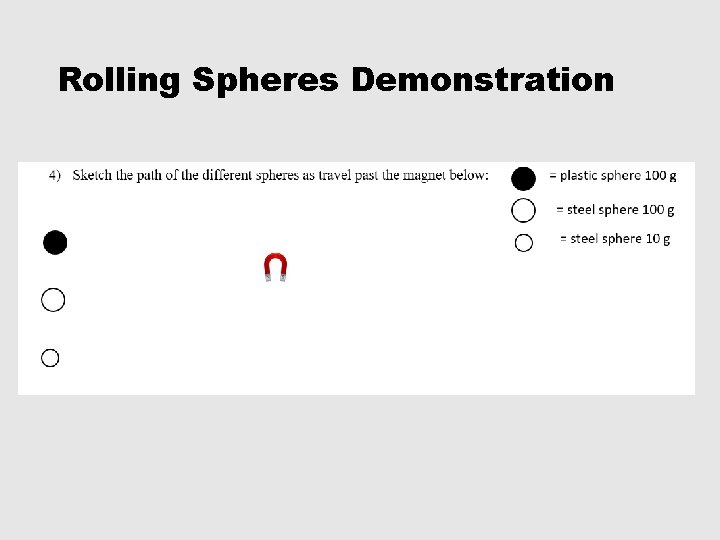
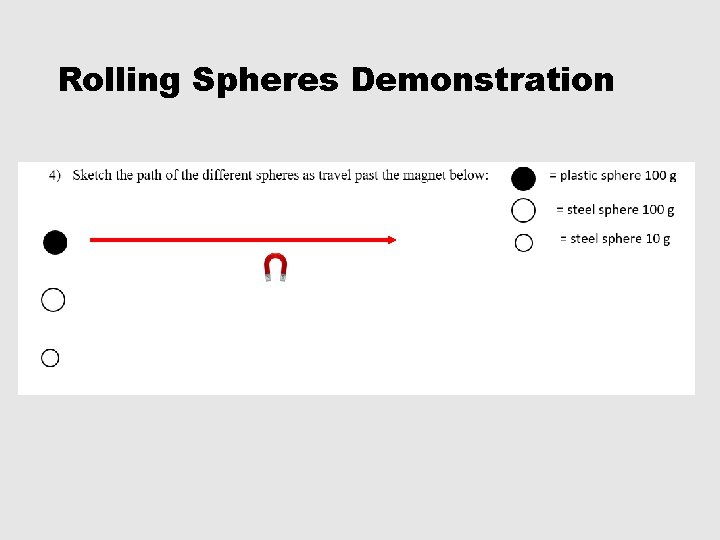
Rolling Spheres Demonstration

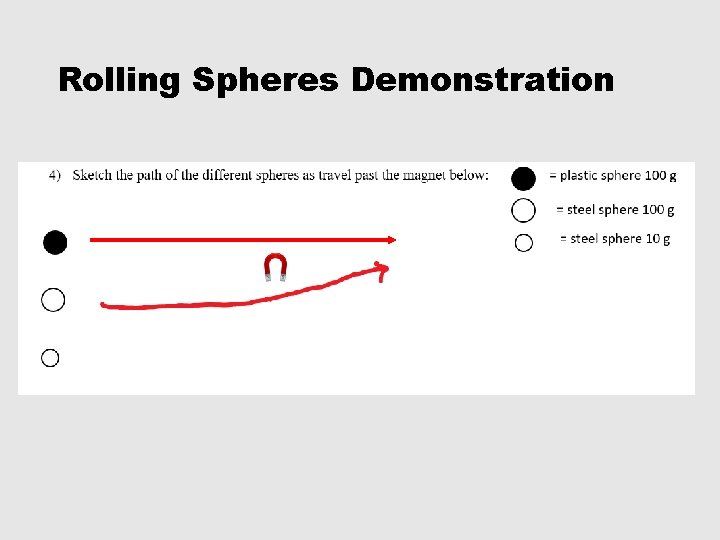
Rolling Spheres Demonstration

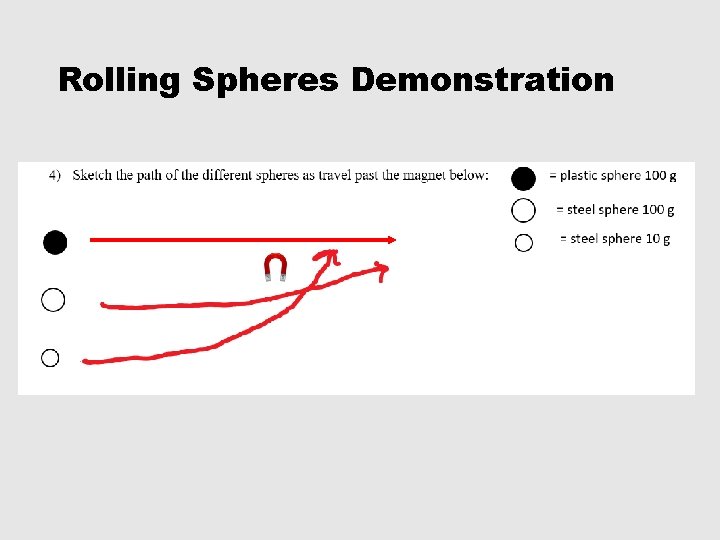
Rolling Spheres Demonstration

Rolling Spheres Demonstration

1 -5, #5 • 5) Which sphere was not deflected at all? Why did it not deflect? Plastic sphere. Plastic is not attracted to a magnet. • 6) Why did the other spheres deflect? All of the other spheres were made of steel which is mostly made of iron. Iron is strongly attracted by a magnet. • 7) What determines the degree of deflection? Which spheres experience the greatest degree of deflection? The degree of deflection is determined by the mass. THE LIGHTER THE MASS THE GREATER THE DEFLECTION!

Rolling Spheres Demonstration • 2 nd Experiment – how does the mass of the cathode ray particle compare to the mass of a atom of the lightest known element? • Is the cathode ray particle an atom of an element? How do you know?

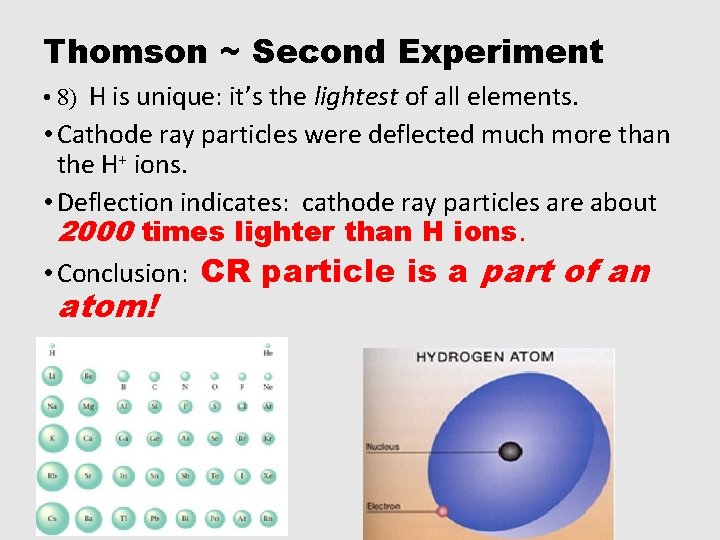
Thomson ~ Second Experiment • 8) H is unique: it’s the lightest of all elements. • Cathode ray particles were deflected much more than the H+ ions. • Deflection indicates: cathode ray particles are about 2000 times lighter than H ions. • Conclusion: CR particle is a part of an atom!

Thomson ~ Third Experiment • Evacuated and filled tube with many elements: all generated cathode ray beams with identical properties. • Conclusion: all elements contain cathode ray particles • 9) Today cathode rays are called ELECTRONS!

Thomson expt video • Summary experiment animation

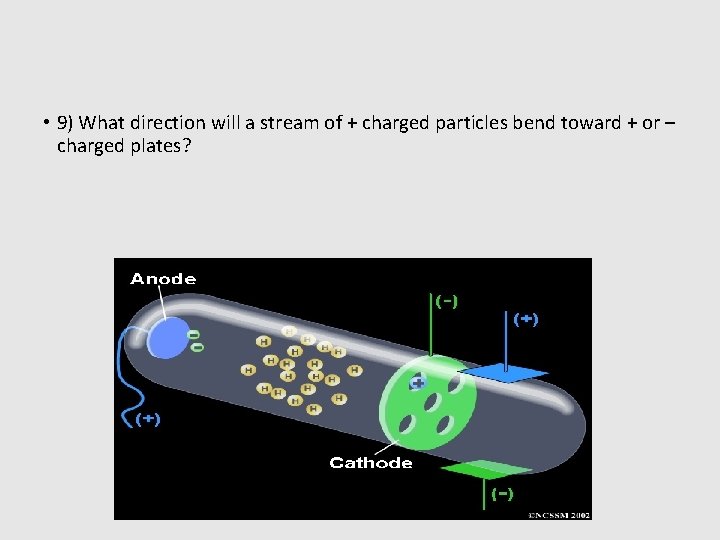
• 9) What direction will a stream of + charged particles bend toward + or – charged plates?

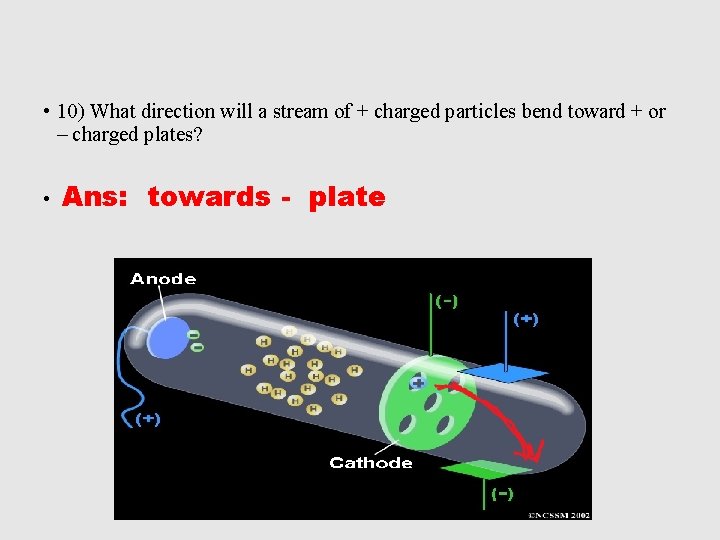
• 10) What direction will a stream of + charged particles bend toward + or – charged plates? • Ans: towards - plate

• 11) What subatomic particle was discovered by experiments similar to those described in question #10? • ANS: The proton.
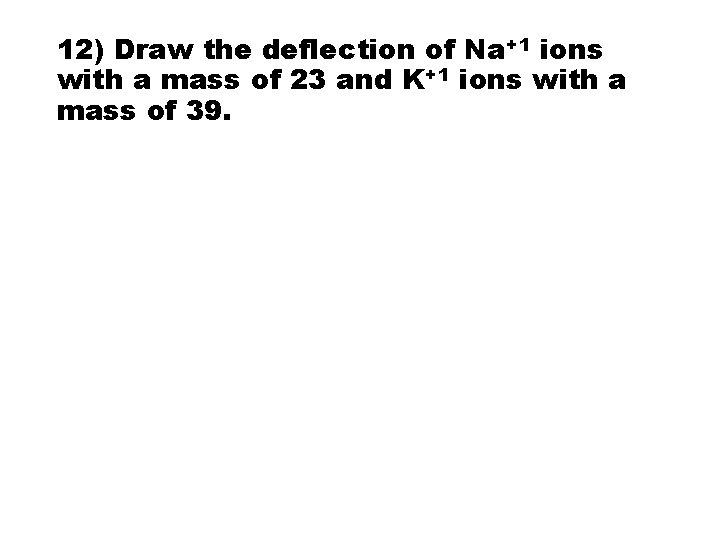
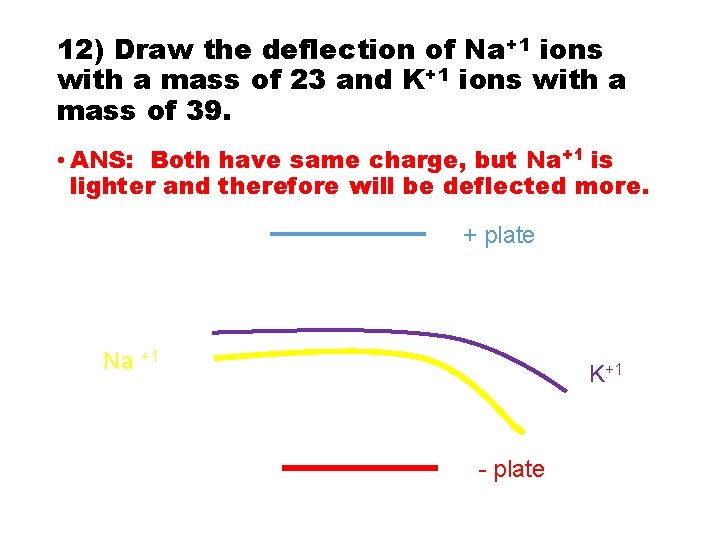
12) Draw the deflection of Na+1 ions with a mass of 23 and K+1 ions with a mass of 39.

12) Draw the deflection of Na+1 ions with a mass of 23 and K+1 ions with a mass of 39. • ANS: Both have same charge, but Na+1 is lighter and therefore will be deflected more. + plate Na +1 K+1 - plate

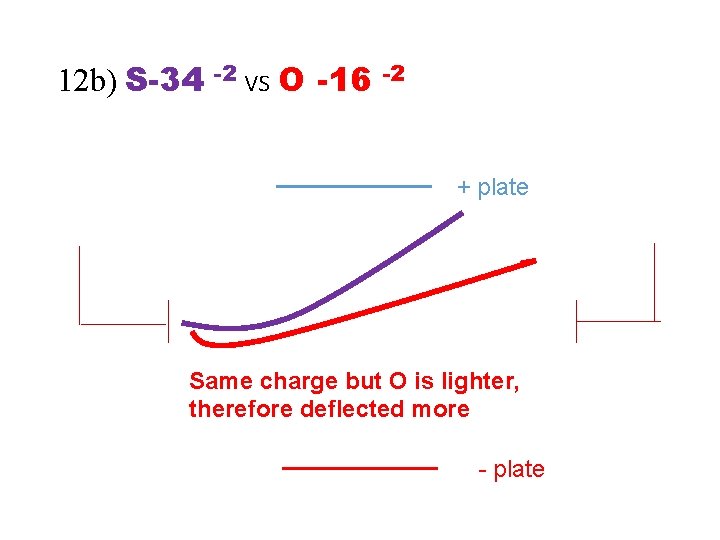
12 b) S-34 -2 vs O -16 -2 + plate - plate

12 b) S-34 -2 vs O -16 -2 + plate Same charge but O is lighter, therefore deflected more - plate

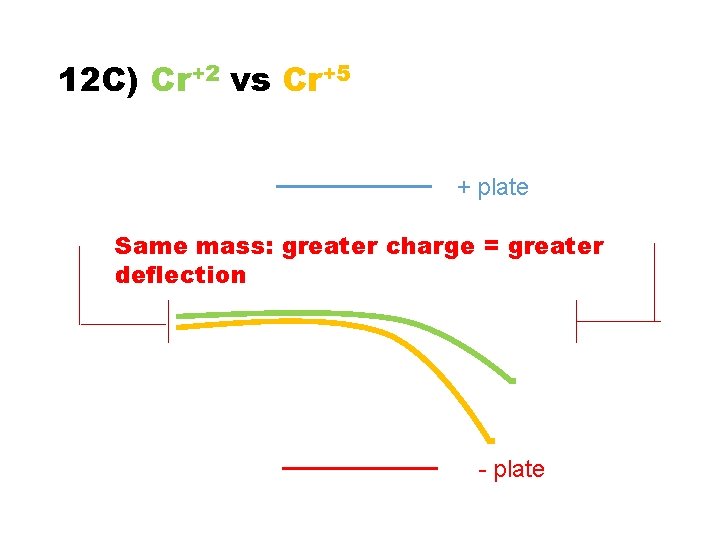
12 C) Cr+2 vs Cr+5 + plate - plate

12 C) Cr+2 vs Cr+5 + plate Same mass: greater charge = greater deflection - plate

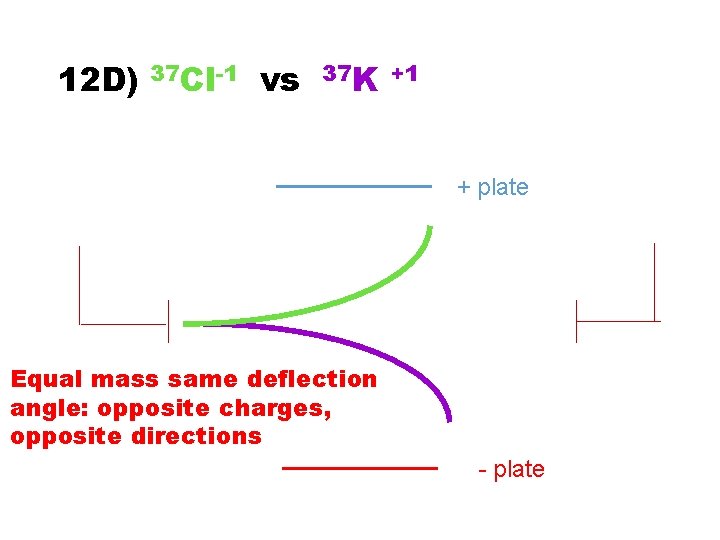
12 D) 37 Cl-1 vs 37 K +1 + plate - plate

12 D) 37 Cl-1 vs 37 K +1 + plate Equal mass same deflection angle: opposite charges, opposite directions - plate