Atomic Structure Chapter 4 Basic Structure of an
Atomic Structure Chapter 4
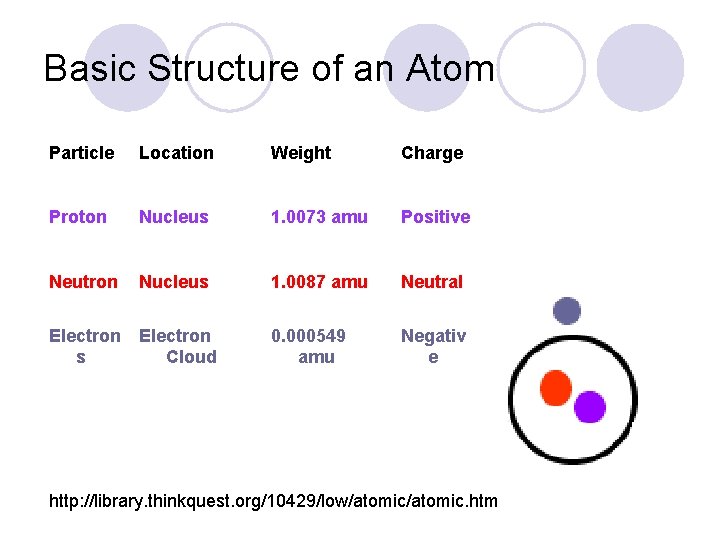
Basic Structure of an Atom Particle Location Weight Charge Proton Nucleus 1. 0073 amu Positive Neutron Nucleus 1. 0087 amu Neutral 0. 000549 amu Negativ e Electron s Cloud http: //library. thinkquest. org/10429/low/atomic. htm
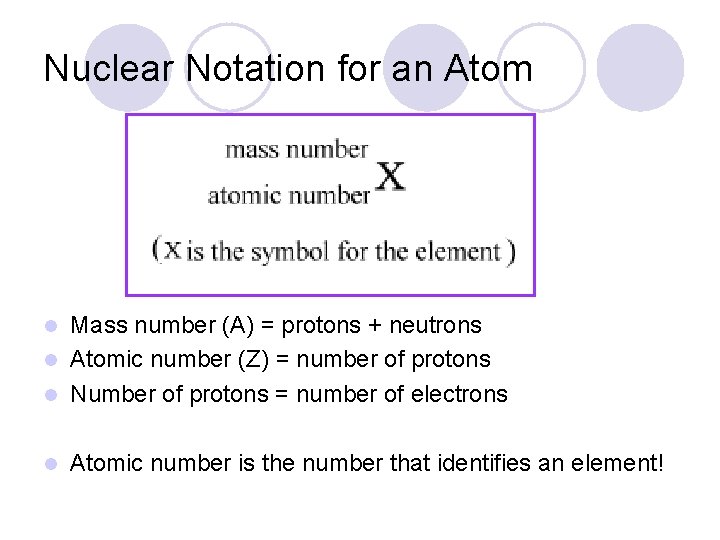
Nuclear Notation for an Atom Mass number (A) = protons + neutrons l Atomic number (Z) = number of protons l Number of protons = number of electrons l l Atomic number is the number that identifies an element!
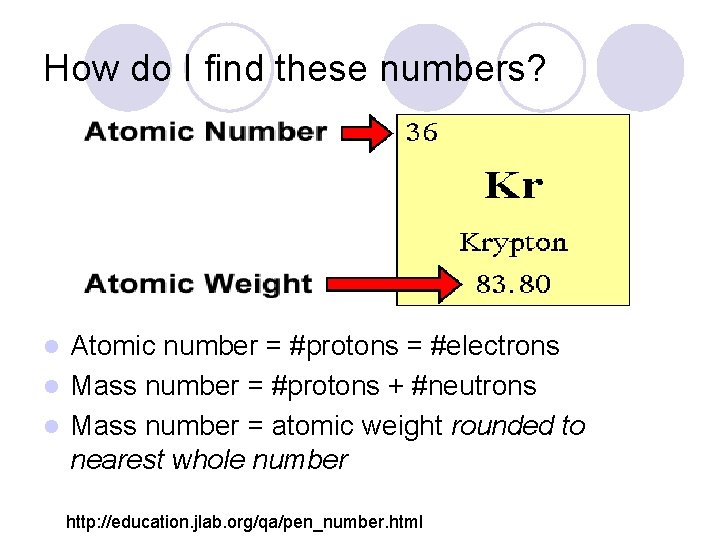
How do I find these numbers? Atomic number = #protons = #electrons l Mass number = #protons + #neutrons l Mass number = atomic weight rounded to nearest whole number l http: //education. jlab. org/qa/pen_number. html
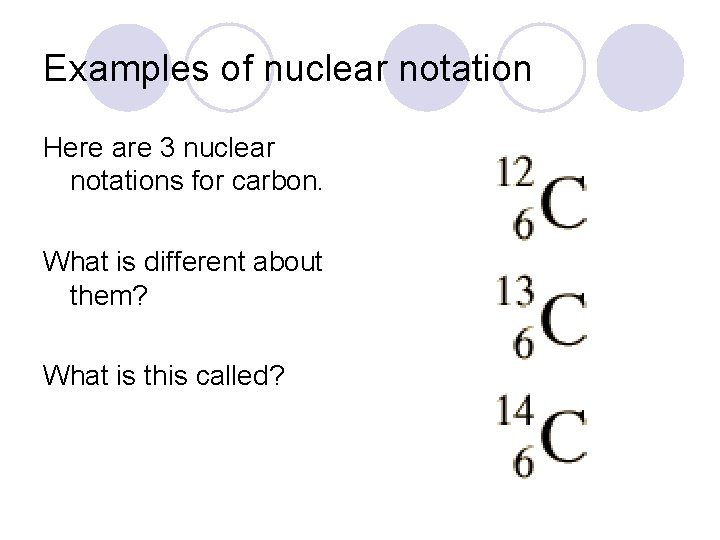
Examples of nuclear notation Here are 3 nuclear notations for carbon. What is different about them? What is this called?
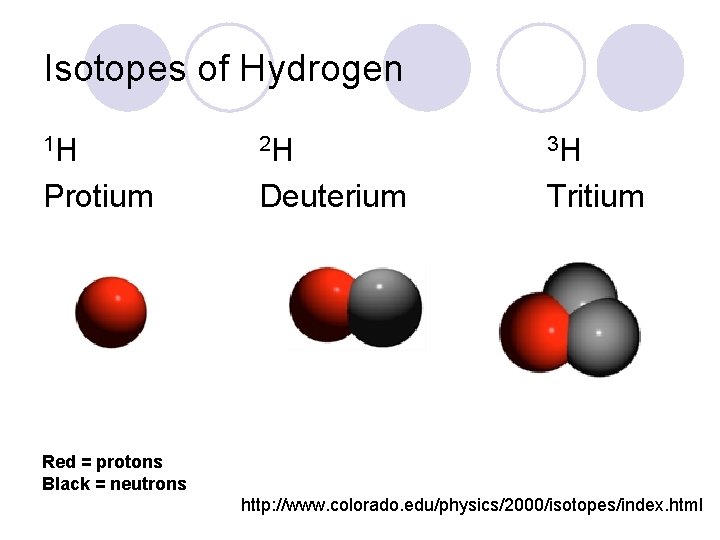
Isotopes of Hydrogen 1 H 2 H 3 H Protium Deuterium Tritium Red = protons Black = neutrons http: //www. colorado. edu/physics/2000/isotopes/index. html
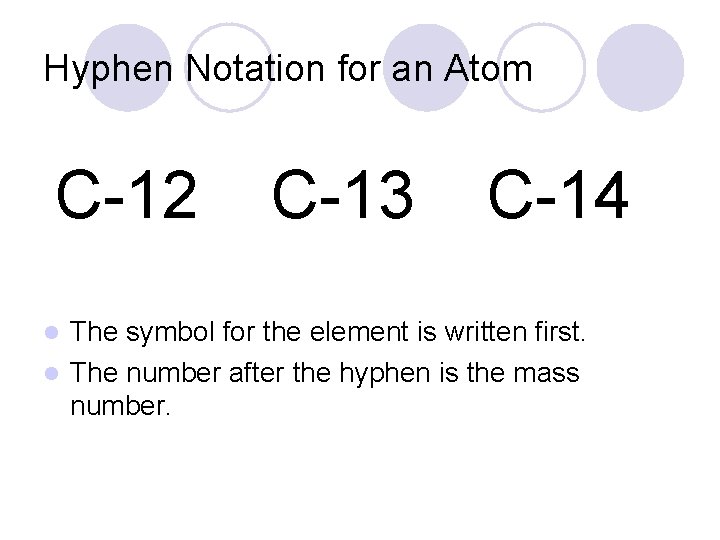
Hyphen Notation for an Atom C-12 C-13 C-14 The symbol for the element is written first. l The number after the hyphen is the mass number. l
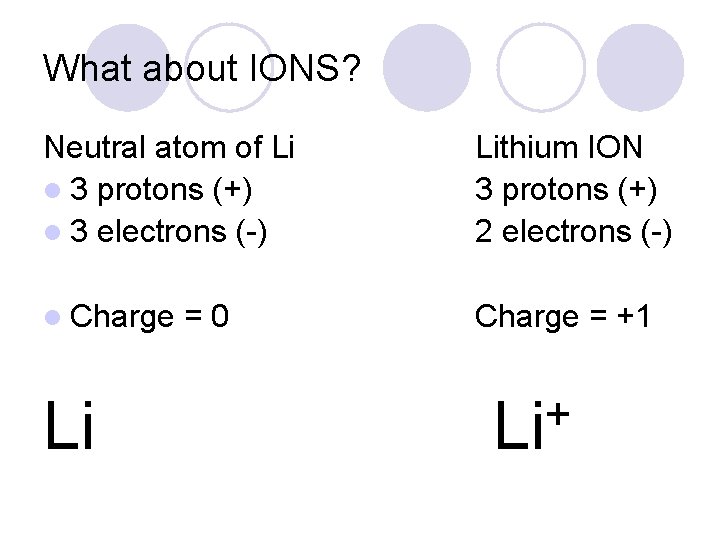
What about IONS? Neutral atom of Li l 3 protons (+) l 3 electrons (-) Lithium ION 3 protons (+) 2 electrons (-) l Charge = +1 Li =0 + Li
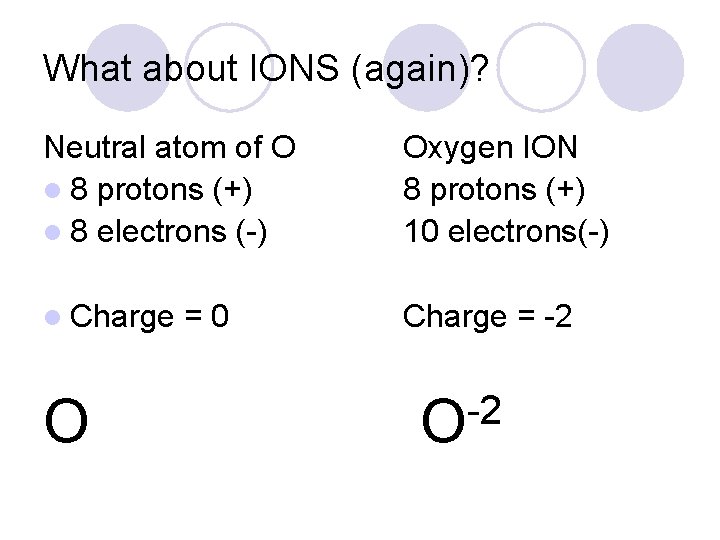
What about IONS (again)? Neutral atom of O l 8 protons (+) l 8 electrons (-) Oxygen ION 8 protons (+) 10 electrons(-) l Charge = -2 O =0 -2 O

2 Types of Ions! l Cation – positive ion ¡ Created when an element loses electrons ¡ Metals l Anion – negative ion ¡ Created when an element gains electrons ¡ Nonmetals l Noble gases (group 18) do NOT form ions
- Slides: 10