Atomic Structure Ch 4 Bohr Model The planetary

Atomic Structure Ch. 4
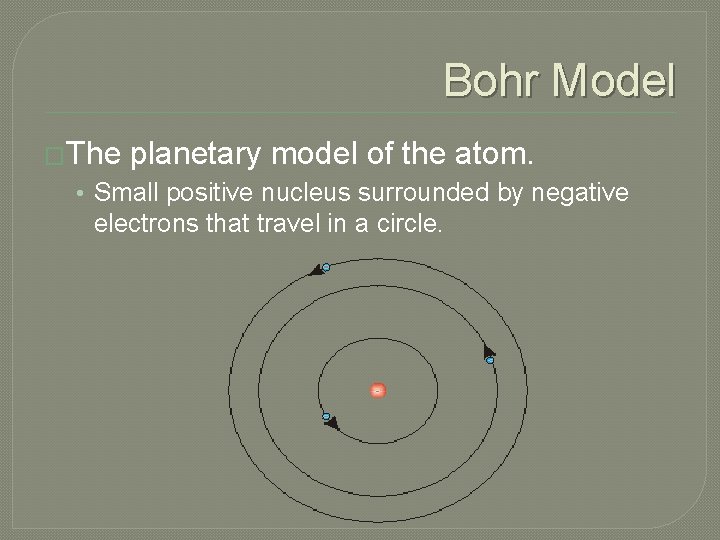
Bohr Model �The planetary model of the atom. • Small positive nucleus surrounded by negative electrons that travel in a circle.

Atomic Structure Definitions �Matter: anything that has mass and volume �Element: a substance that cannot be broken down into simpler substances. �Atom: the smallest unit of matter and the smallest particle of an element.
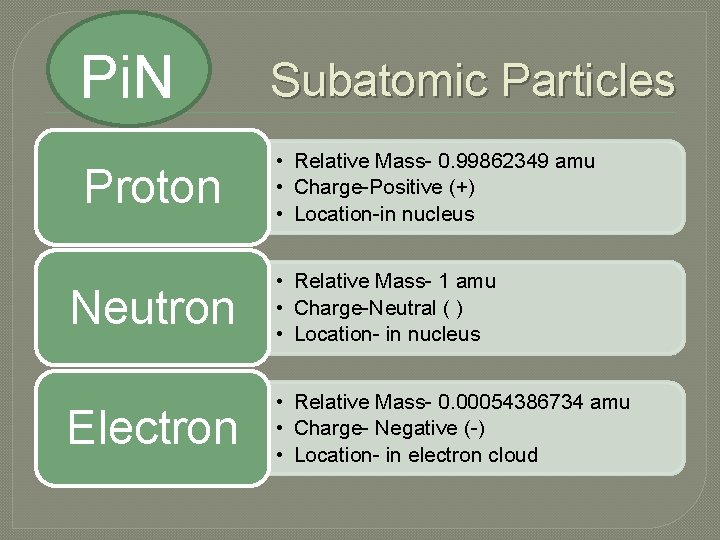
Pi. N Subatomic Particles Proton • Relative Mass- 0. 99862349 amu • Charge-Positive (+) • Location-in nucleus Neutron • Relative Mass- 1 amu • Charge-Neutral ( ) • Location- in nucleus Electron • Relative Mass- 0. 00054386734 amu • Charge- Negative (-) • Location- in electron cloud
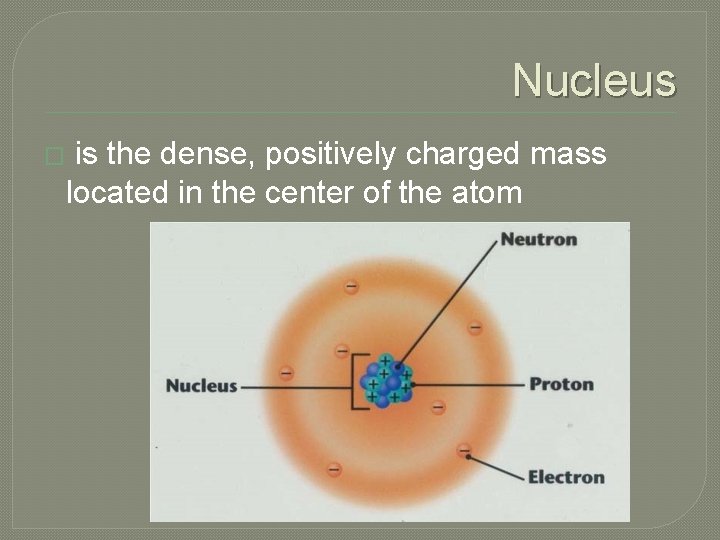
Nucleus � is the dense, positively charged mass located in the center of the atom
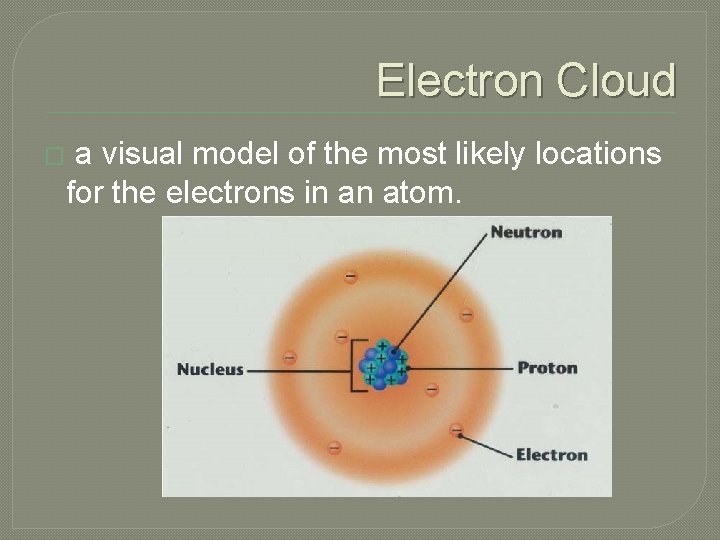
Electron Cloud � a visual model of the most likely locations for the electrons in an atom.
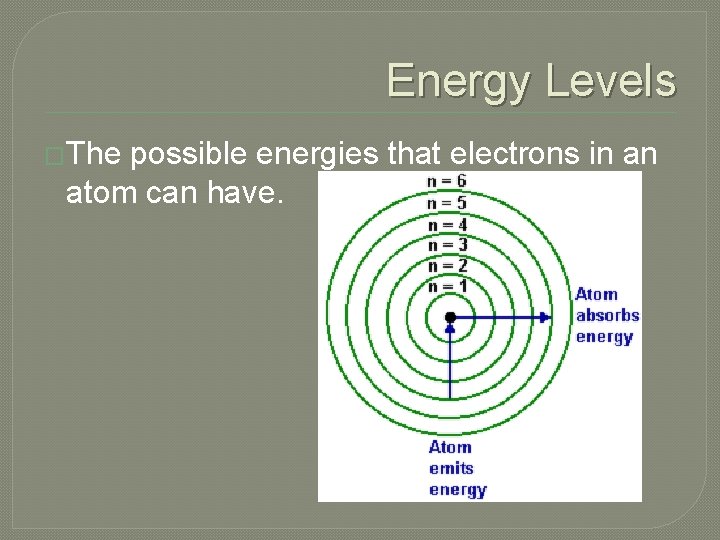
Energy Levels �The possible energies that electrons in an atom can have.
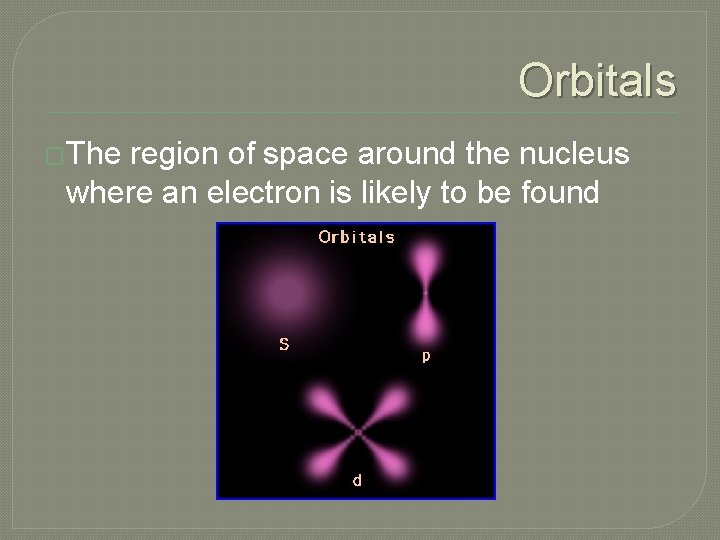
Orbitals �The region of space around the nucleus where an electron is likely to be found

Important Definitions �Stable- has 2 or 8 valence electrons, does not want to bond to other atoms. �Ground state- a state in which all the electrons in an atoms have the lowest possible energies. �Valence electrons-an electron that is in the highest energy level of an atom. (The outside energy shell)
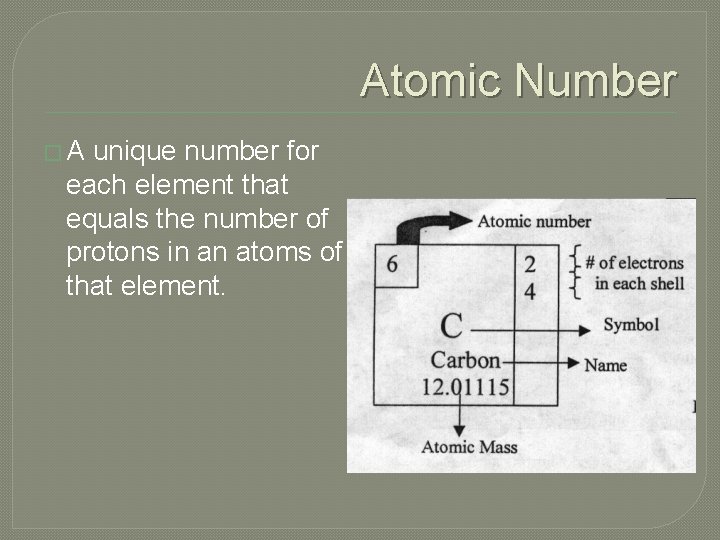
Atomic Number �A unique number for each element that equals the number of protons in an atoms of that element.
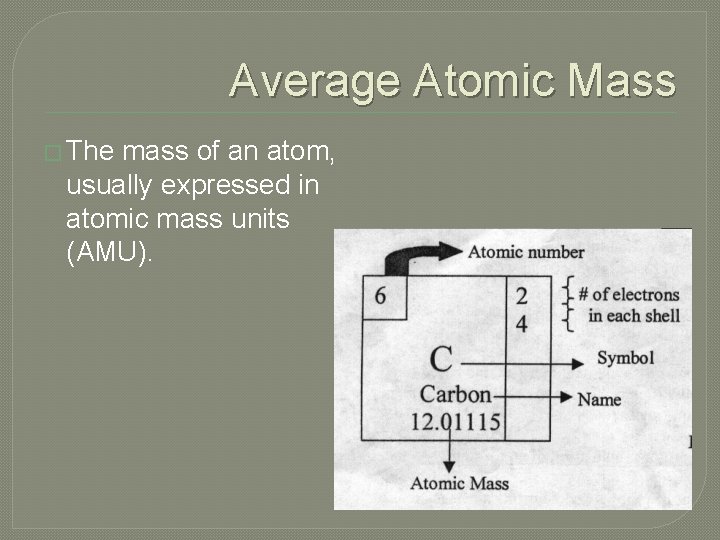
Average Atomic Mass � The mass of an atom, usually expressed in atomic mass units (AMU).
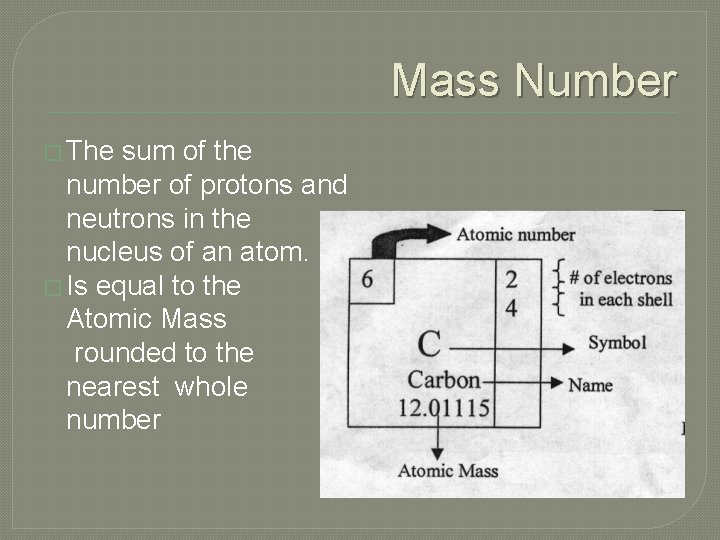
Mass Number � The sum of the number of protons and neutrons in the nucleus of an atom. � Is equal to the Atomic Mass rounded to the nearest whole number
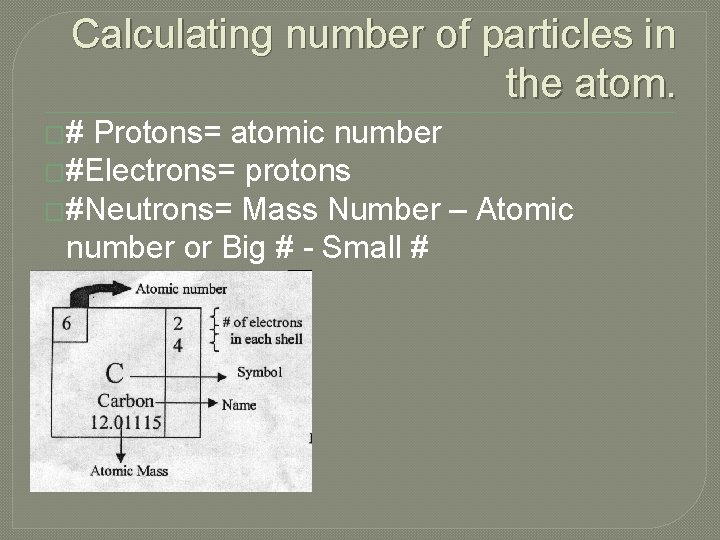
Calculating number of particles in the atom. �# Protons= atomic number �#Electrons= protons �#Neutrons= Mass Number – Atomic number or Big # - Small #
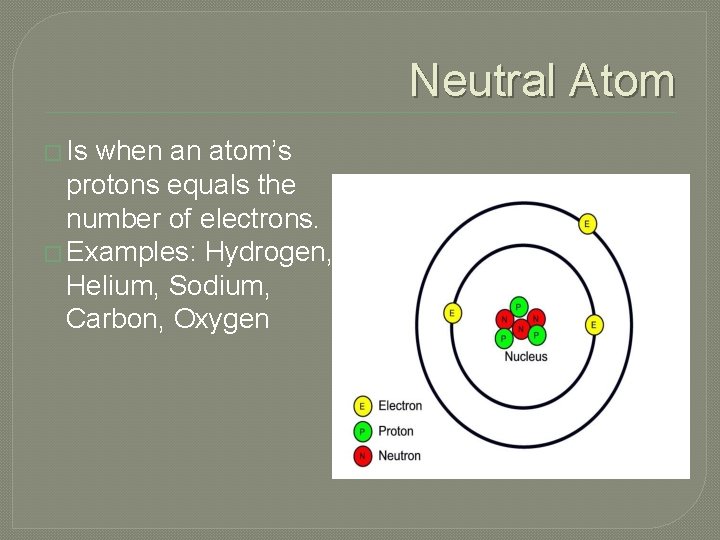
Neutral Atom � Is when an atom’s protons equals the number of electrons. � Examples: Hydrogen, Helium, Sodium, Carbon, Oxygen

Textbook Resource � You can use the text as a reference to this. Chapter 4 is on atomic structure and may be useful if you have trouble with some of the concepts. You can access the book using the instructions on the right. � HOW TO ACCESS THE TEXTBOOK ONLINE � Go to www. pearsonsuccess net. com � Username: East. Bears � Password: boyertown#1 � Click on prentice hall physical science link
- Slides: 15