ATOMIC STRUCTURE BUTTONS Click here Clicking here will
ATOMIC STRUCTURE BUTTONS Click here; Clicking here will allow you tocommon hear some information onhere the Clicking here will take you to ayou ion table. Clicking here will take to aback periodic table, clicking here Clicking here will reveal some information. Clicking here will move you to the next Clicking here will reveal an answer. Clicking here will bring you topage. this page. Clicking here will move you back a page. topic. Clicking here again willpage stop the sound. again will take you back toback the were on previously. again will take you to theyou previous page. TOPICS Jump to… …what’s in an atom. Jump to… …electron shells. Jump to… …mass and atomic numbers. Jump to… …building atoms and ions (3 pages). Jump to… …isotopes. Jump to… …practice questions 1 and 2 (two pages). Jump to… …exam questions 1 and 2 (four pages).
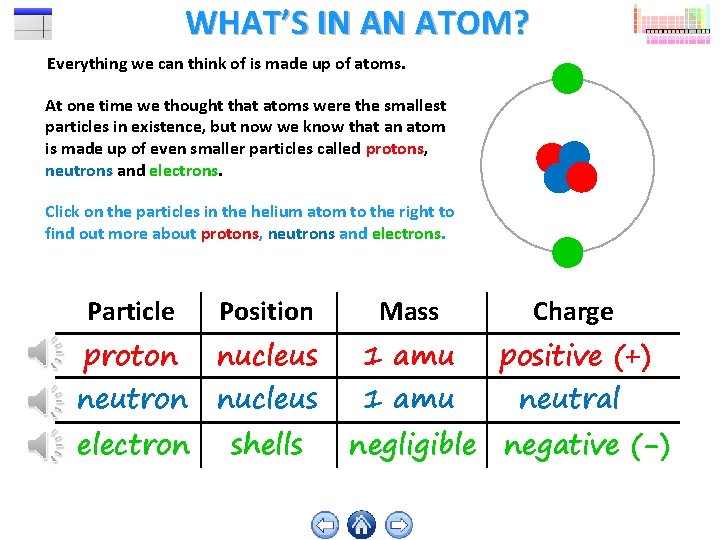
WHAT’S IN AN ATOM? Everything we can think of is made up of atoms. At one time we thought that atoms were the smallest particles in existence, but now we know that an atom is made up of even smaller particles called protons, neutrons and electrons. Click on the particles in the helium atom to the right to find out more about protons, neutrons and electrons. Particle Position Mass Charge proton nucleus 1 amu positive (+) electron shells neutron nucleus 1 amu neutral negligible negative (-)

ELECTRON SHELLS The electrons surround the nucleus in electron shells. These electron shells are sometimes called orbitals or energy levels. Click below to see how many electrons can be placed in each shell. First Shell - Second Shell Third Shell - - NUCLEUS - - -
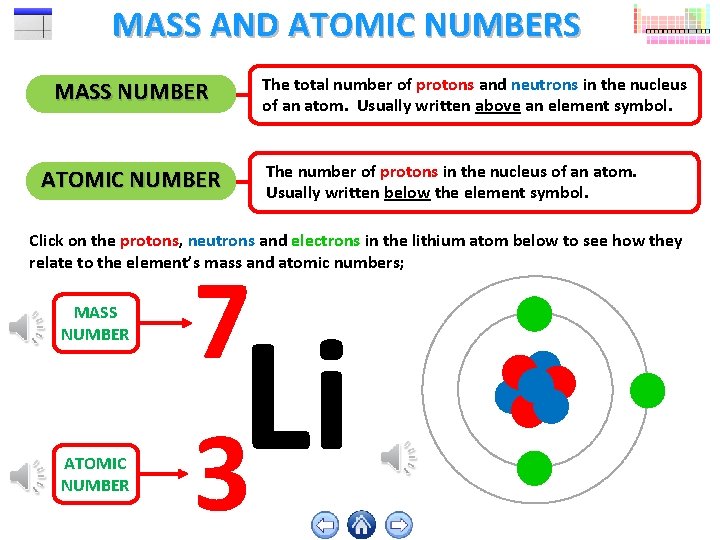
MASS AND ATOMIC NUMBERS MASS NUMBER ATOMIC NUMBER The total number of protons and neutrons in the nucleus of an atom. Usually written above an element symbol. The number of protons in the nucleus of an atom. Usually written below the element symbol. Click on the protons, neutrons and electrons in the lithium atom below to see how they relate to the element’s mass and atomic numbers; MASS NUMBER ATOMIC NUMBER 7 Li 3
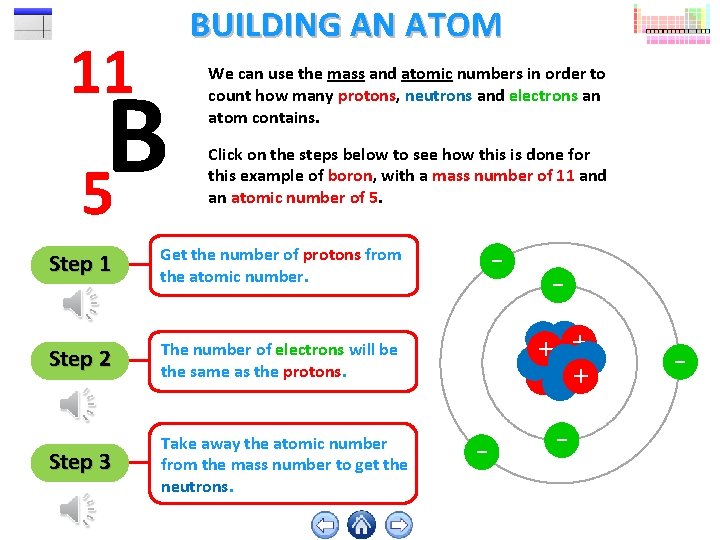
11 BUILDING AN ATOM B 5 We can use the mass and atomic numbers in order to count how many protons, neutrons and electrons an atom contains. Click on the steps below to see how this is done for this example of boron, with a mass number of 11 and an atomic number of 5. Step 1 Get the number of protons from the atomic number. Step 2 The number of electrons will be the same as the protons. Step 3 Take away the atomic number from the mass number to get the neutrons. - + ++ - - -
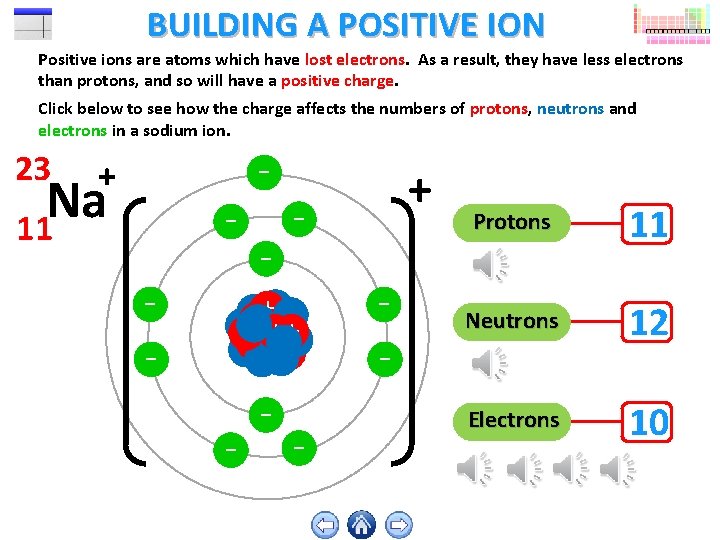
BUILDING A POSITIVE ION Positive ions are atoms which have lost electrons. As a result, they have less electrons than protons, and so will have a positive charge. Click below to see how the charge affects the numbers of protons, neutrons and electrons in a sodium ion. 23 + Na 11 - - - ++ ++++ ++ - - Protons 11 Neutrons 12 Electrons 10
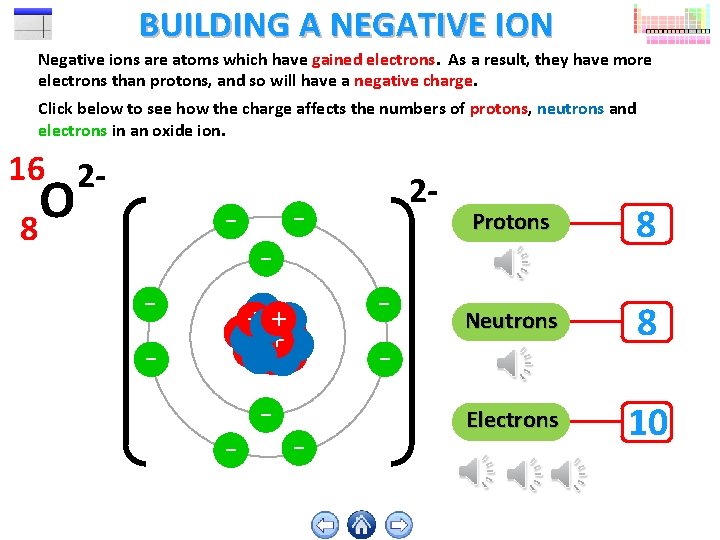
BUILDING A NEGATIVE ION Negative ions are atoms which have gained electrons. As a result, they have more electrons than protons, and so will have a negative charge. Click below to see how the charge affects the numbers of protons, neutrons and electrons in an oxide ion. 16 2 O 8 - - - + ++ - - 2 - - - Protons 8 Neutrons 8 Electrons 10
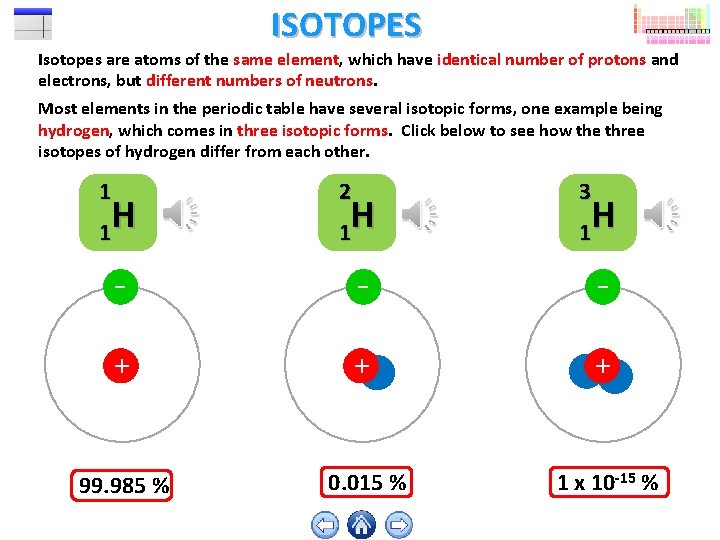
ISOTOPES Isotopes are atoms of the same element, which have identical number of protons and electrons, but different numbers of neutrons. Most elements in the periodic table have several isotopic forms, one example being hydrogen, which comes in three isotopic forms. Click below to see how the three isotopes of hydrogen differ from each other. 1 H 1 2 H 1 3 H 1 - - - + + + 99. 985 % 0. 015 % 1 x 10 -15 %
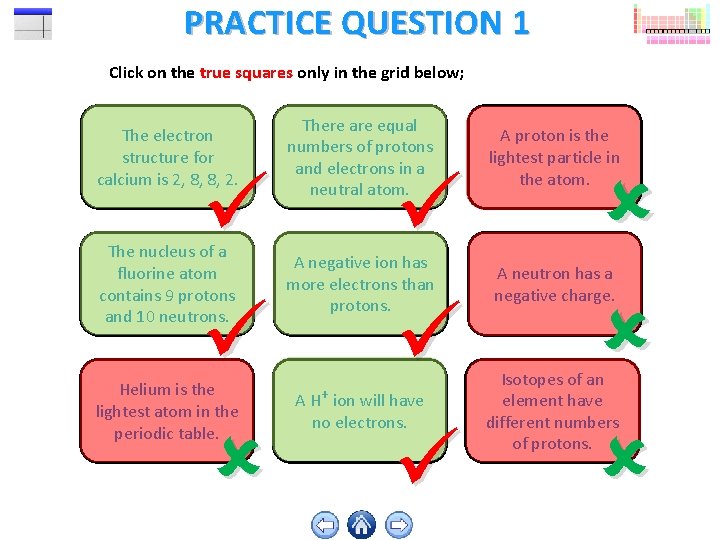
PRACTICE QUESTION 1 Click on the true squares only in the grid below; The electron structure for calcium is 2, 8, 8, 2. There are equal numbers of protons and electrons in a neutral atom. A proton is the lightest particle in the atom. The nucleus of a fluorine atom contains 9 protons and 10 neutrons. A negative ion has more electrons than protons. A neutron has a negative charge. Helium is the lightest atom in the periodic table. A H+ ion will have no electrons. Isotopes of an element have different numbers of protons.
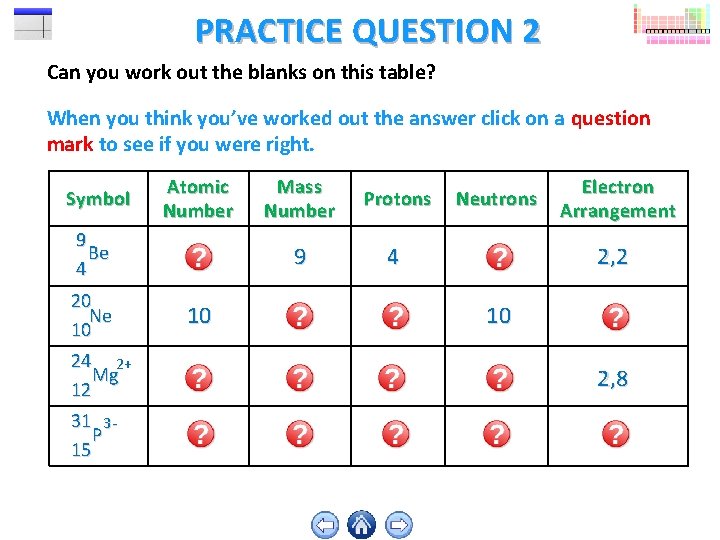
PRACTICE QUESTION 2 Can you work out the blanks on this table? When you think you’ve worked out the answer click on a question mark to see if you were right. Symbol Atomic Number Mass Number Protons Neutrons Electron Arrangement 9 Be 4 4 9 4 5 2, 2 20 Ne 10 10 20 10 10 2, 8 24 2+ Mg 12 12 24 12 12 2, 8 31 3 P 15 15 31 15 16 2, 8, 8
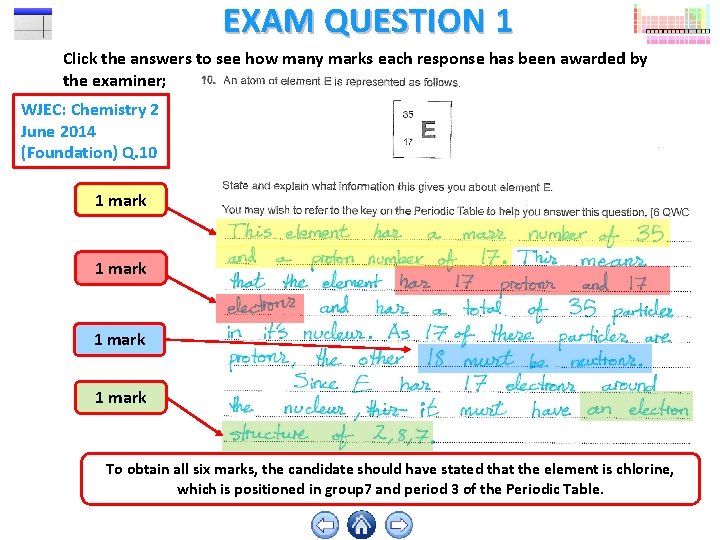
EXAM QUESTION 1 Click the answers to see how many marks each response has been awarded by the examiner; WJEC: Chemistry 2 June 2014 (Foundation) Q. 10 1 mark To obtain all six marks, the candidate should have stated that the element is chlorine, which is positioned in group 7 and period 3 of the Periodic Table.
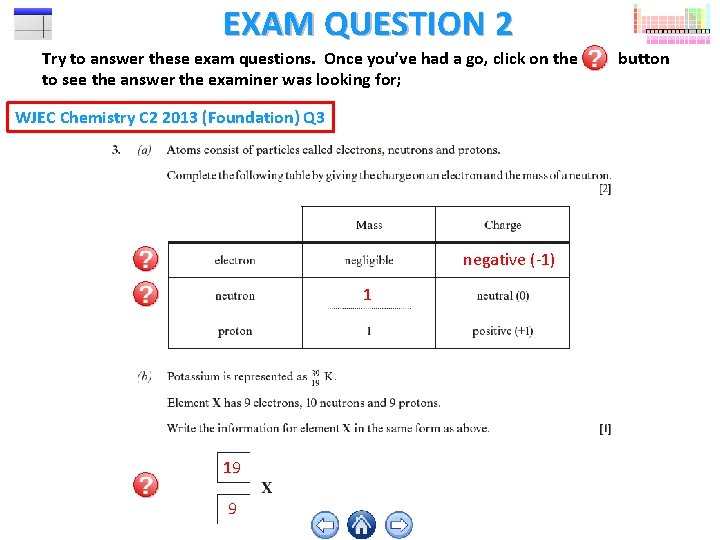
EXAM QUESTION 2 Try to answer these exam questions. Once you’ve had a go, click on the to see the answer the examiner was looking for; WJEC Chemistry C 2 2013 (Foundation) Q 3 negative (-1) 1 19 9 button
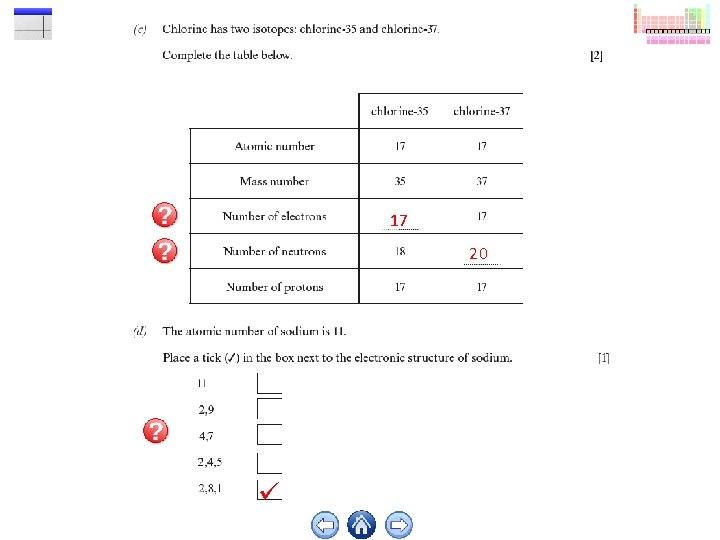
17 20
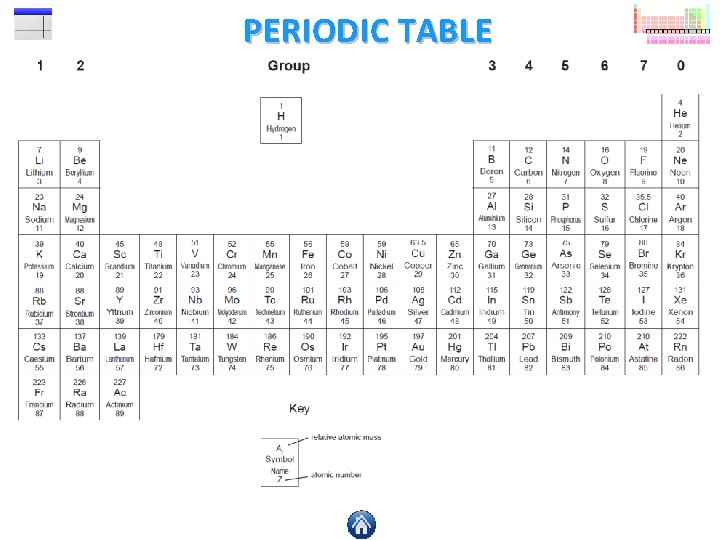
PERIODIC TABLE
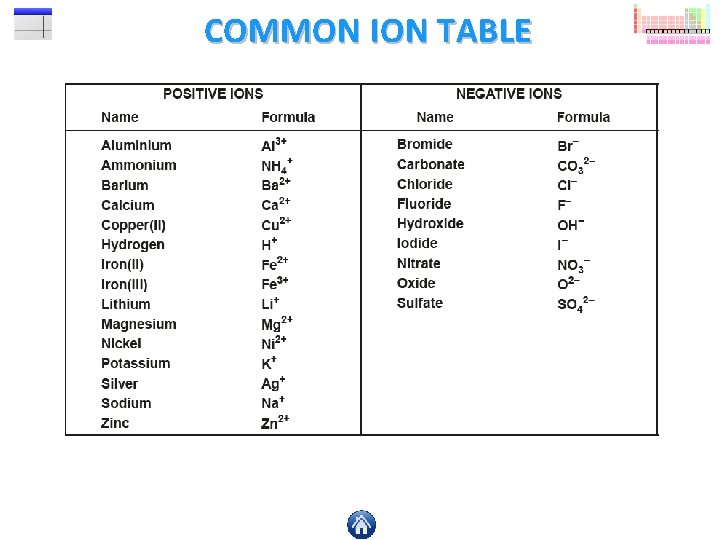
COMMON ION TABLE
- Slides: 15