Atomic Structure Atoms True or False An atom














- Slides: 37

Atomic Structure

Atoms True or False: An atom is the smallest particle of an element that retains its identity in a chemical reaction. • True • False

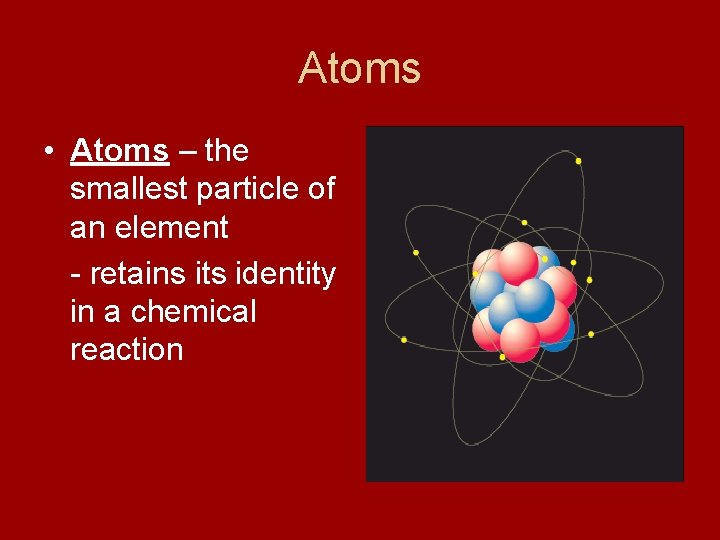
Atoms • Atoms – the smallest particle of an element - retains its identity in a chemical reaction

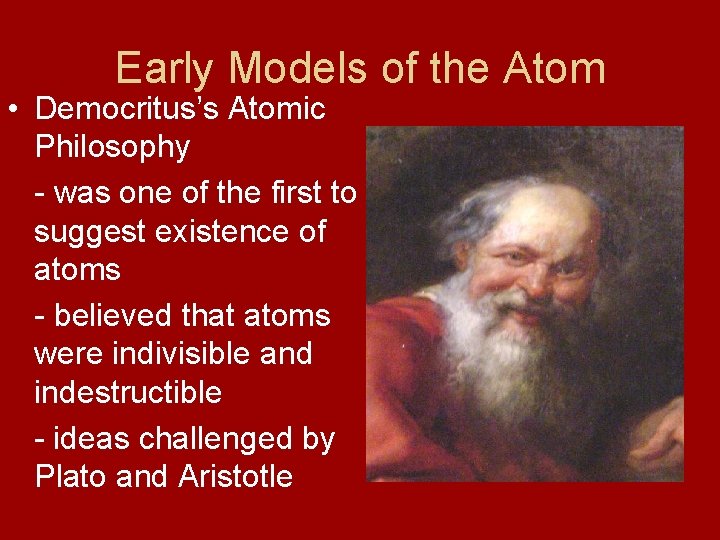
Early Models of the Atom • Democritus’s Atomic Philosophy - was one of the first to suggest existence of atoms - believed that atoms were indivisible and indestructible - ideas challenged by Plato and Aristotle

Dalton’s Atomic Theory • Dalton transformed Democritus’s ideas on atoms into a scientific theory - studied ratios in which elements combine in chemical reactions

Dalton’s Atomic Theory 1. All elements composed of tiny indivisible particles called atoms. 2. Atoms of same element are identical. (Each element has a different type of atom. ) 3. Atoms of different elements can physically mix together or form compounds in whole-number ratios. 4. Chemical reactions occur when atoms are separated, joined, or rearranged. Atoms of one element never change into atoms of another element due to chemical reactions.

Sizing up the Atom • Radii of most atoms fall within range of 5 X 10 -11 m to 2 X 10 -10 m • Atoms can still be observed - by scanning tunneling microscopes - atomic scale = “nanoscale”

Sizing up the Atom

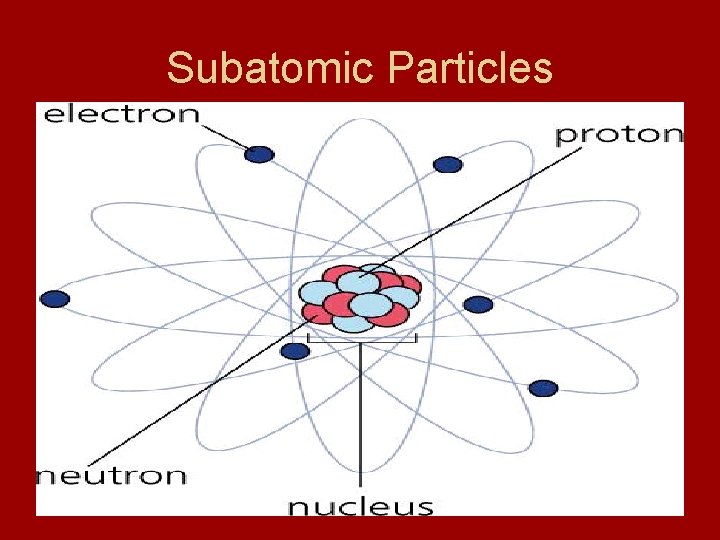
Subatomic Particles • Most of Dalton’s atomic theory is still accepted today - the major change is that atoms are known to be divisible - divide into electrons, protons and neutrons

Subatomic Particles

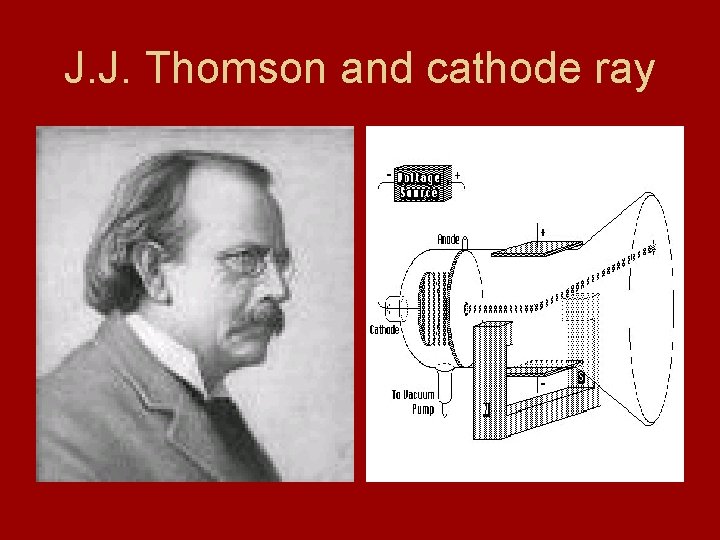
Electrons • Electron – negatively charged subatomic particles - discovered by J. J Thomson in 1897 - passed electric current through gas at low pressure - he sealed gas in tubes with electrodes at both ends - anode = positive charge - cathode = negative charge - cathode ray - glowing beam that traveled from cathode to anode

Electrons • Thomson noted that cathode ray is deflected by a magnet • Positive charge = attracts cathode ray • Negative charge = repels • Opposite charges attract and like repel • Hypothesized cathode ray is a stream of tiny negatively charged particles - originally called corpuscles - now called electrons

J. J. Thomson and cathode ray

Robert A. Millikan • Discovered electron has one unit of negative charge - also discovered mass is of electron is 1/1840 of that of a Hydrogen atom

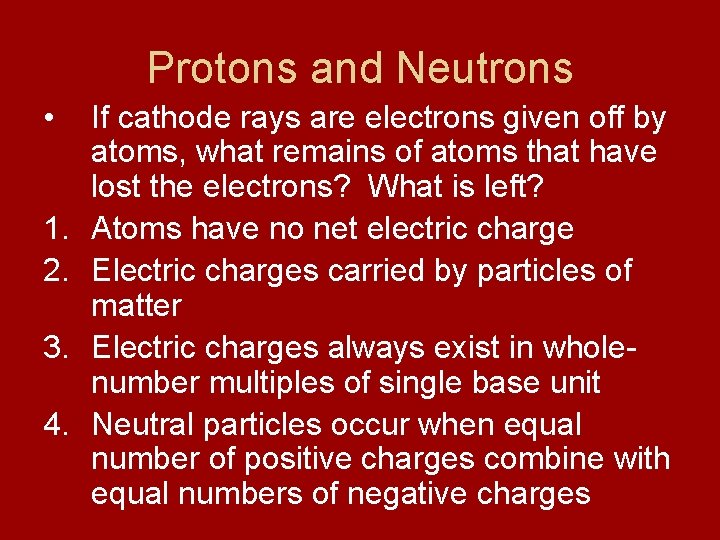
Protons and Neutrons • 1. 2. 3. 4. If cathode rays are electrons given off by atoms, what remains of atoms that have lost the electrons? What is left? Atoms have no net electric charge Electric charges carried by particles of matter Electric charges always exist in wholenumber multiples of single base unit Neutral particles occur when equal number of positive charges combine with equal numbers of negative charges

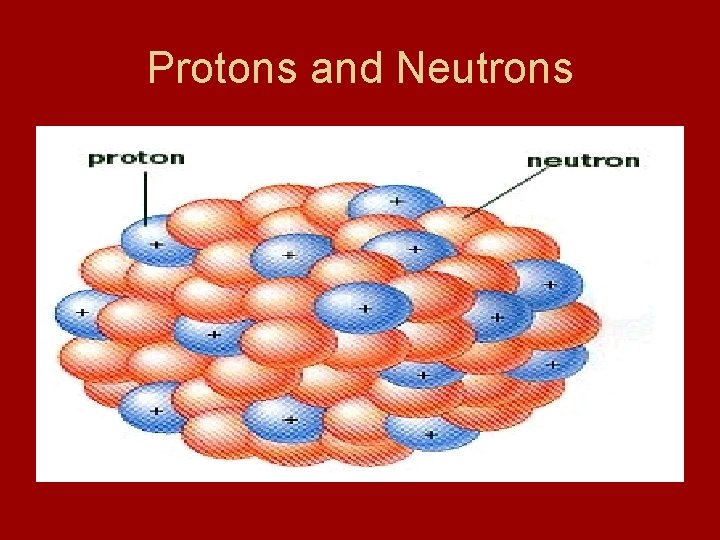
Protons • Protons – positively charged subatomic particles - has a mass of 1840 times that of electron - discovered by Eugen Goldstein after observing a cathode-ray tube - found rays traveling in direction opposite to that of cathode rays - concluded these to be positive

Neutrons • Neutrons – subatomic particles with no charge - mass nearly equal to that of a proton - discovered by English physicist James Chadwick in 1932

Protons and Neutrons

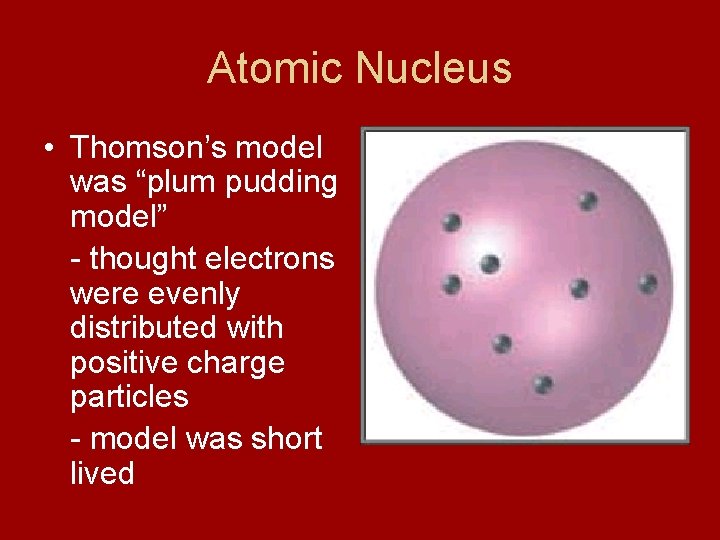
Atomic Nucleus • Thomson’s model was “plum pudding model” - thought electrons were evenly distributed with positive charge particles - model was short lived

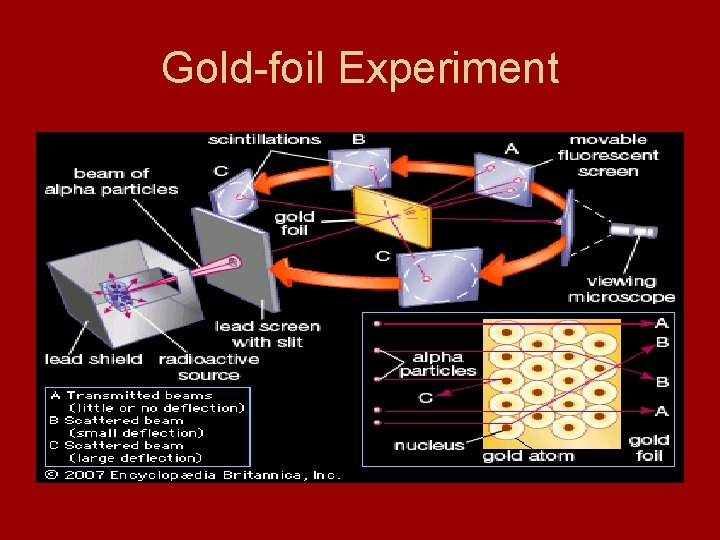
Ernest Rutherford • Ernest Rutherford – disproved “plum pudding model” - used “gold-foil experiment

Atomic Nucleus • “Gold-foil experiment” - directed beam of positively charged alpha particles at thin gold foil - most particles passed through - only a few deflected - discovered positive charges in dense core of atom - nucleus – tiny central core of an atom that is composed of protons and neutrons - electrons surround nucleus in electron cloud - 10, 000 times small than radius of overall atom

Gold-foil Experiment

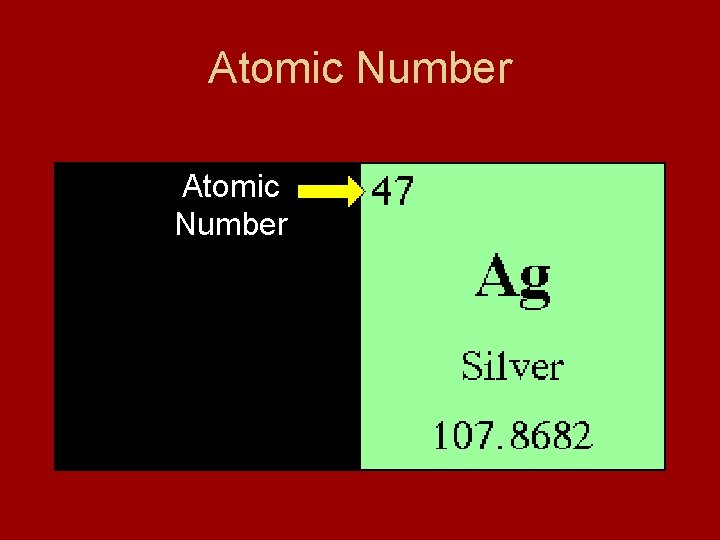
Atomic Number • Elements differ because they contain different numbers of protons • Atomic Number – number of protons in nucleus of an atom of that element - identifies an element - number of protons = number of electrons - electrically neutral

Atomic Number

Mass Number • Most of an atoms mass is concentrated in its nucleus • Mass Number – total number of protons and neutrons in an atom • Number of neutrons in an atom is difference between mass number and atomic number • # neutrons=mass number - atomic number

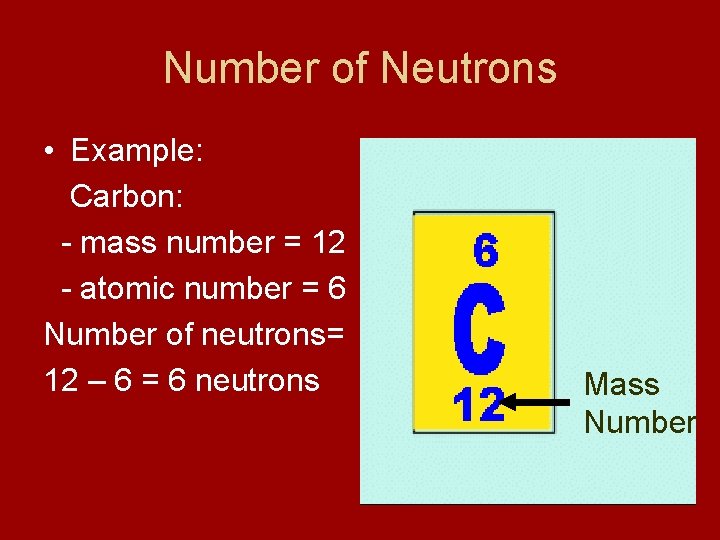
Number of Neutrons • Example: Carbon: - mass number = 12 - atomic number = 6 Number of neutrons= 12 – 6 = 6 neutrons Mass Number

Examples • • Atomic # Mass # Beryllium (Be) 4 9 Neon (Ne) 10 20 Sodium (Na) 11 23 How many electrons, protons, and neutrons in each? Be = 4 e, 4 p, 5 n Ne = 10 e, 10 p, 10 n Na = 11 e, 11 p, 12 n

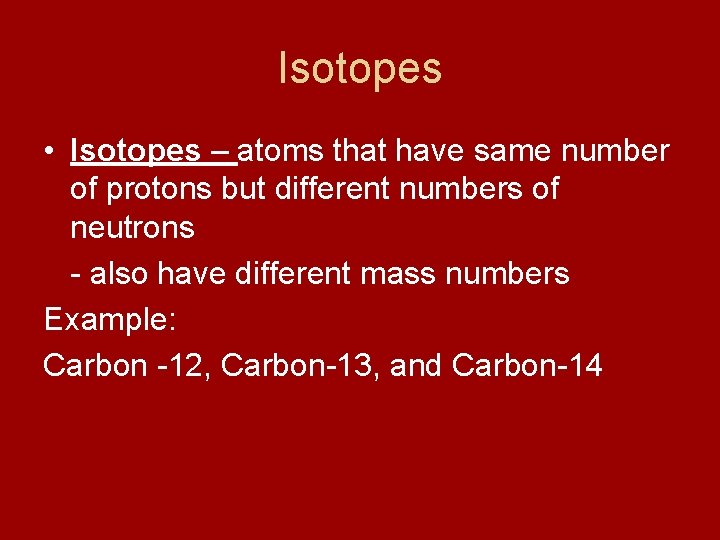
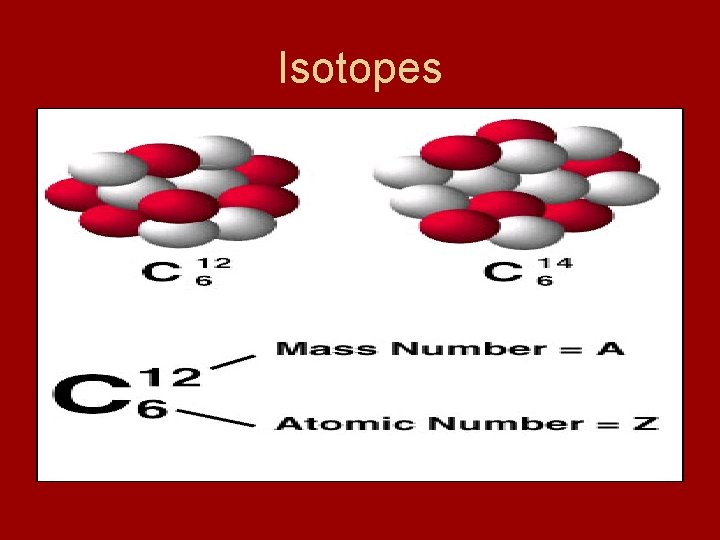
Isotopes • Isotopes – atoms that have same number of protons but different numbers of neutrons - also have different mass numbers Example: Carbon -12, Carbon-13, and Carbon-14

Isotopes

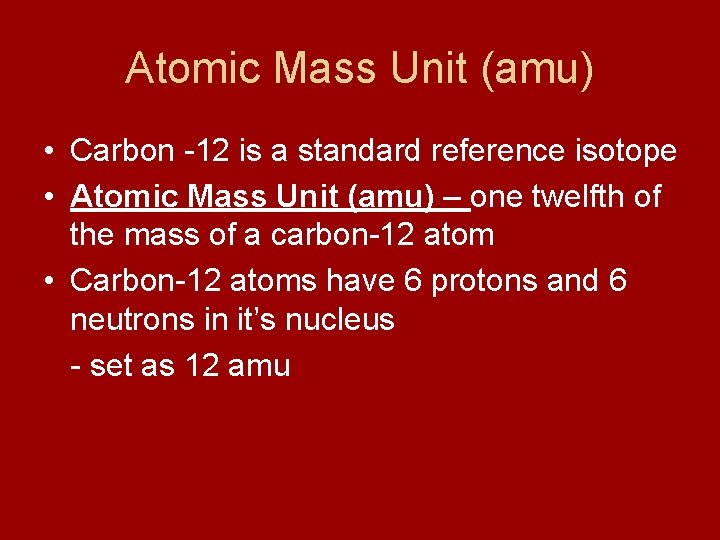
Atomic Mass Unit (amu) • Carbon -12 is a standard reference isotope • Atomic Mass Unit (amu) – one twelfth of the mass of a carbon-12 atom • Carbon-12 atoms have 6 protons and 6 neutrons in it’s nucleus - set as 12 amu

Atomic Mass Unit

Atomic Mass • Mass of atoms depends on number of protons and neutrons in nucleus - in most cases atomic mass is not a whole number - most elements occur as a mixture of two or more isotopes • Atomic Mass – weighted average mass of atoms in a naturally occurring sample of the element - reflects both mass and relative abundance of isotopes as occur in nature

Calculating Average Atomic Mass • To calculate amu: 1. multiply mass of each isotope by its natural abundance (expressed as a decimal) 2. Add the products

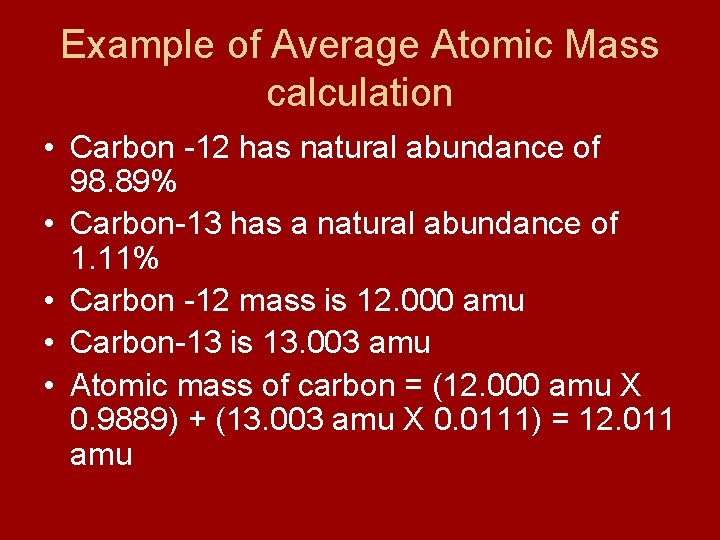
Example of Average Atomic Mass calculation • Carbon -12 has natural abundance of 98. 89% • Carbon-13 has a natural abundance of 1. 11% • Carbon -12 mass is 12. 000 amu • Carbon-13 is 13. 003 amu • Atomic mass of carbon = (12. 000 amu X 0. 9889) + (13. 003 amu X 0. 0111) = 12. 011 amu

Practice calculating atomic mass • Copper has 2 isotopes Copper-63 and Copper-65 • Copper-63 has an abundance of 69. 2% and an amu of 62. 93 • Copper-65 has an abundance of 30. 8% and an amu of 64. 93 • Calculate the average atomic mass • Answer = 63. 546 amu

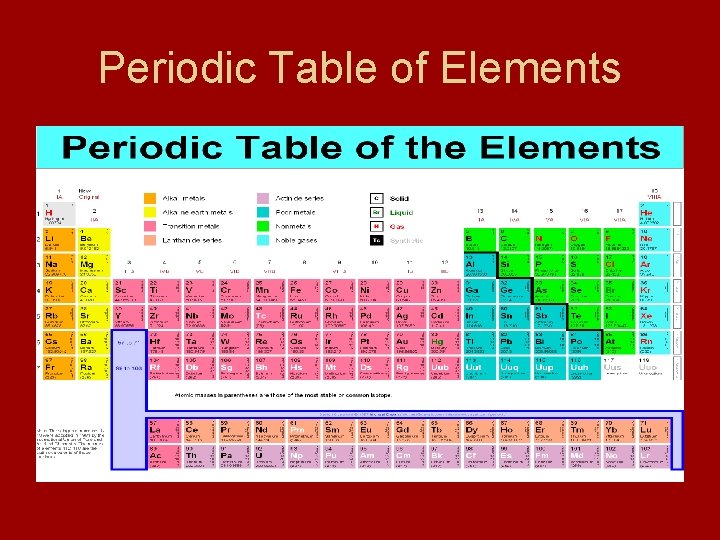
Periodic Table Preview • Periodic Table – arrangement of elements in which elements - separated into groups based on set of repeating properties - allows you to compare properties of one element to another • Period – each horizontal row • Group – each vertical column

Periodic Table of Elements