Atomic Structure Atom Basic unit of matter Made
Atomic Structure
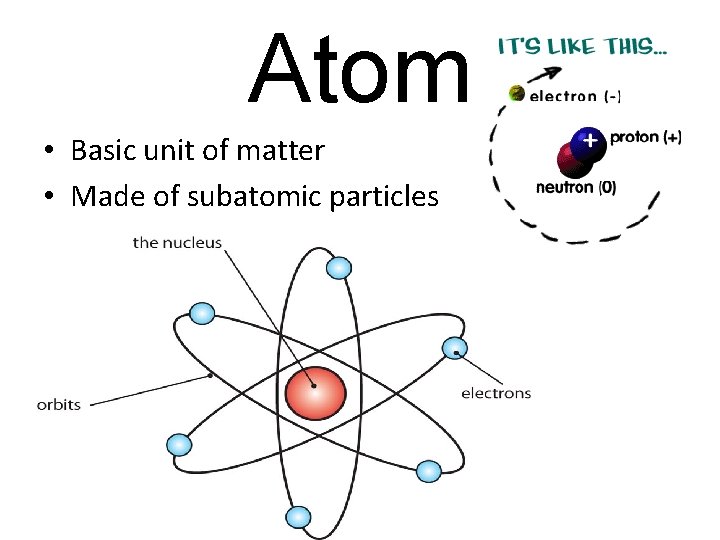
Atom • Basic unit of matter • Made of subatomic particles
Element • Substance made of same type of atom
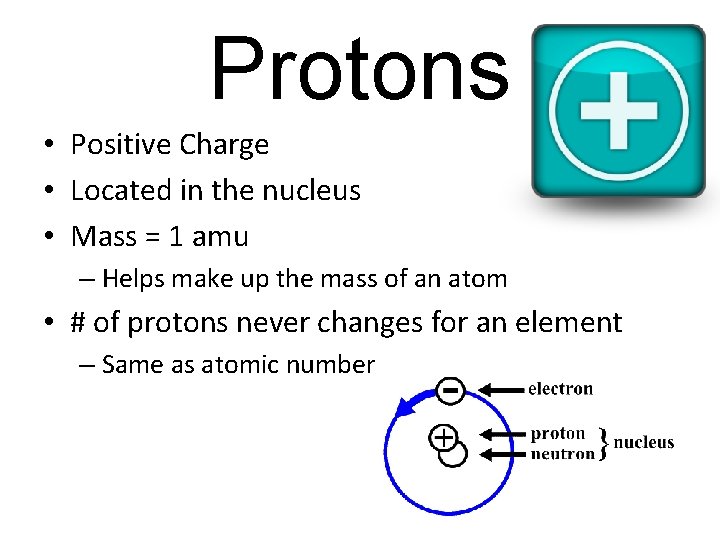
Protons • Positive Charge • Located in the nucleus • Mass = 1 amu – Helps make up the mass of an atom • # of protons never changes for an element – Same as atomic number
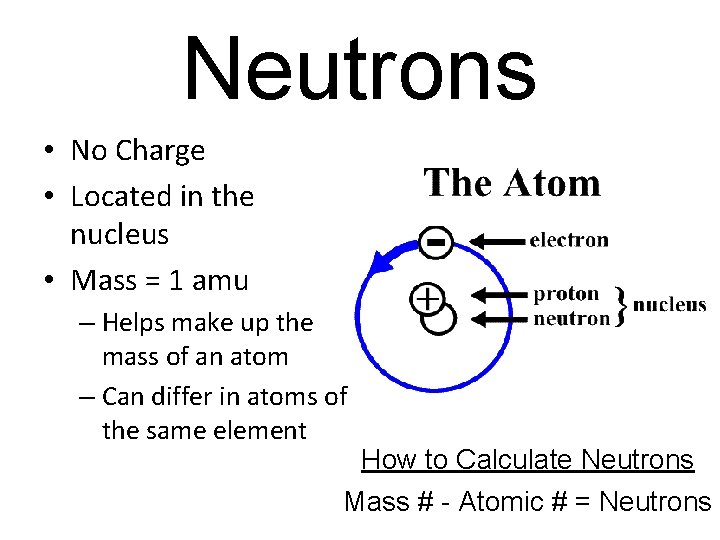
Neutrons • No Charge • Located in the nucleus • Mass = 1 amu – Helps make up the mass of an atom – Can differ in atoms of the same element How to Calculate Neutrons Mass # - Atomic # = Neutrons
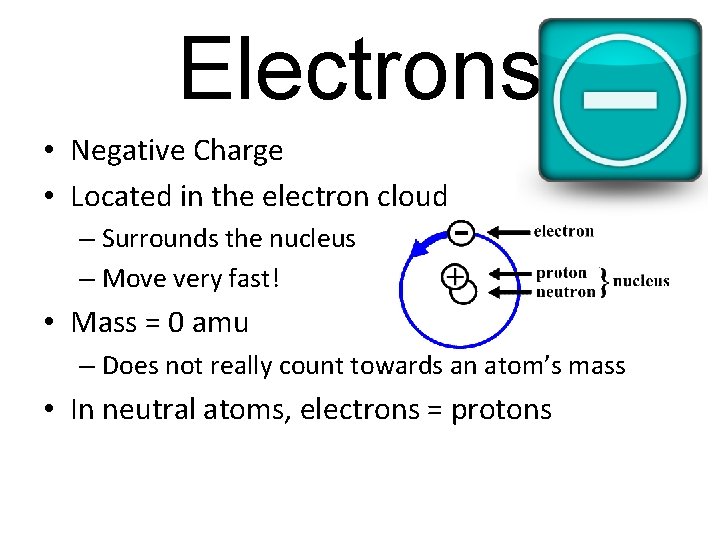
Electrons • Negative Charge • Located in the electron cloud – Surrounds the nucleus – Move very fast! • Mass = 0 amu – Does not really count towards an atom’s mass • In neutral atoms, electrons = protons
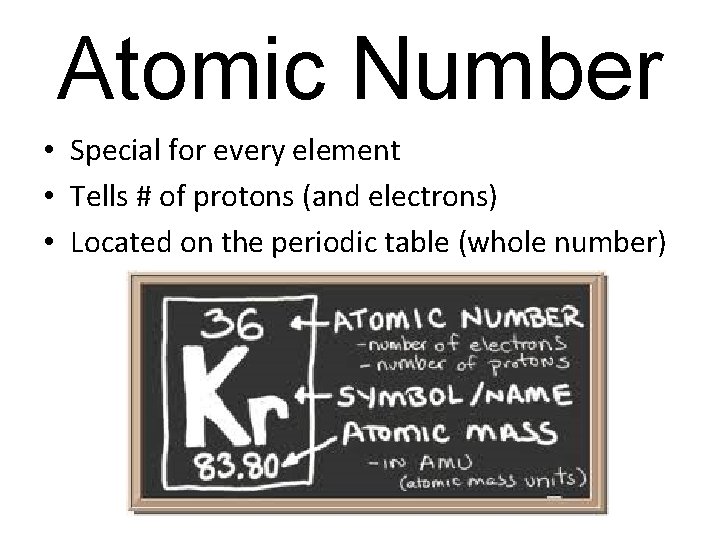
Atomic Number • Special for every element • Tells # of protons (and electrons) • Located on the periodic table (whole number)
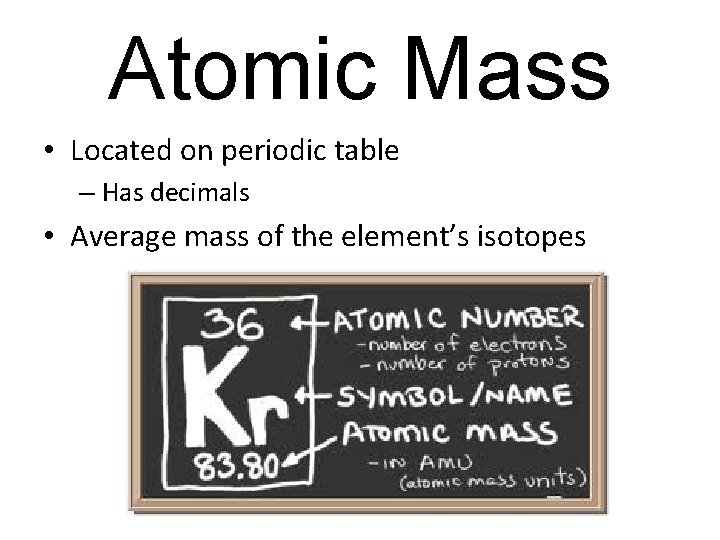
Atomic Mass • Located on periodic table – Has decimals • Average mass of the element’s isotopes
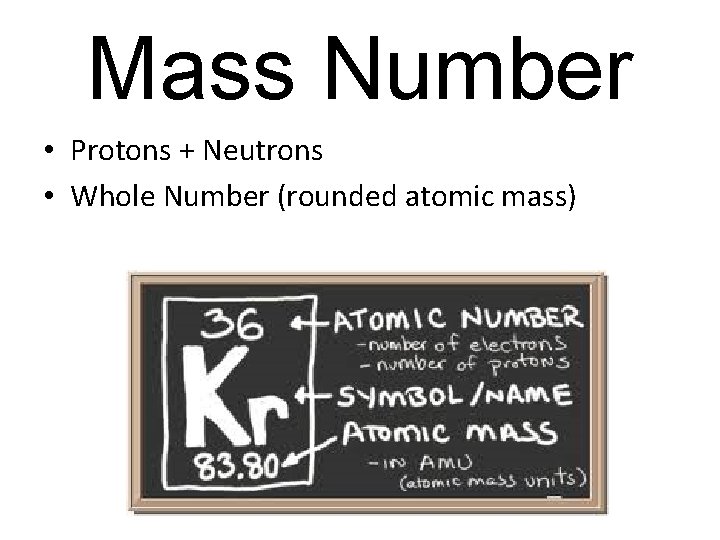
Mass Number • Protons + Neutrons • Whole Number (rounded atomic mass)

Practice • How many protons does nitrogen have?

Practice • What is the atomic mass of calcium?

Practice • What is the mass number of calcium?

Practice • How many neutrons are in chlorine?
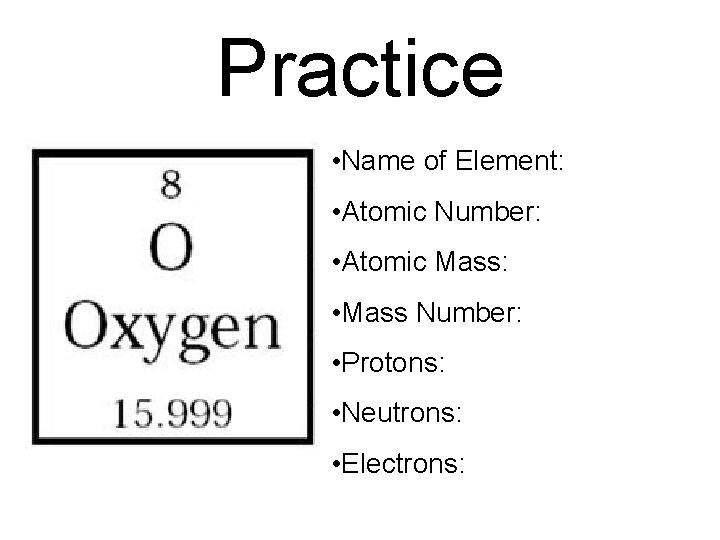
Practice • Name of Element: • Atomic Number: • Atomic Mass: • Mass Number: • Protons: • Neutrons: • Electrons:
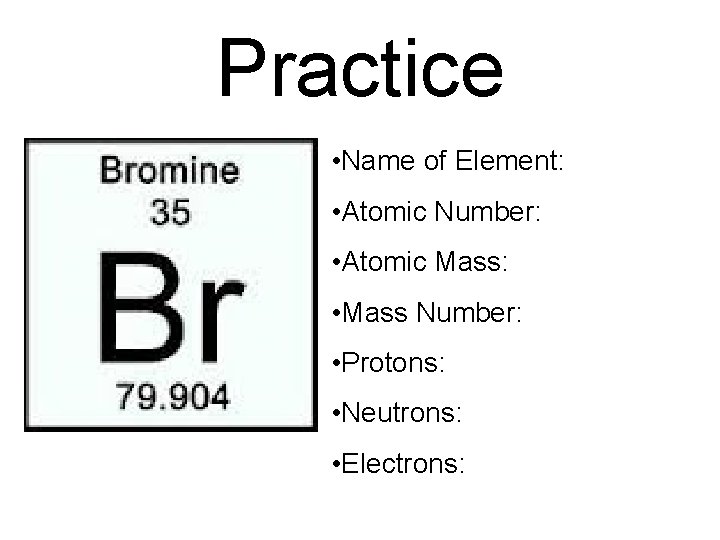
Practice • Name of Element: • Atomic Number: • Atomic Mass: • Mass Number: • Protons: • Neutrons: • Electrons:
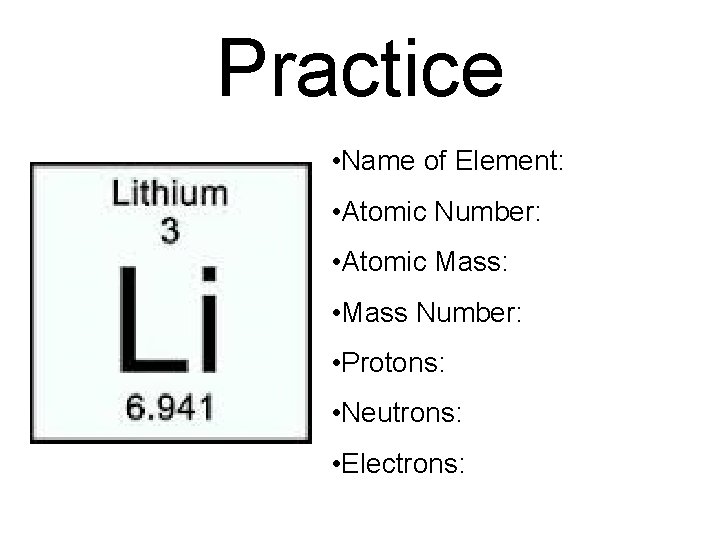
Practice • Name of Element: • Atomic Number: • Atomic Mass: • Mass Number: • Protons: • Neutrons: • Electrons:
Just How Small Are Atoms?
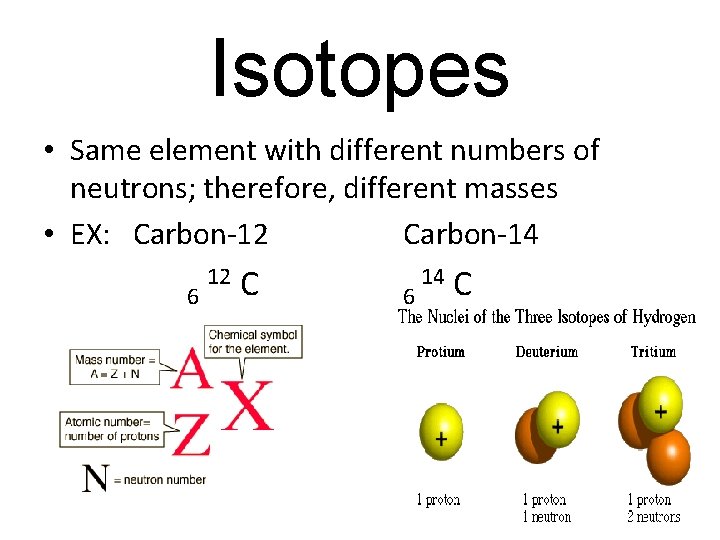
Isotopes • Same element with different numbers of neutrons; therefore, different masses • EX: Carbon-12 Carbon-14 6 12 C 6 14 C

Practice • How many neutrons in Potassium-39 Potassium-41 Fluorine-19 Fluorine-18

Practice • Ni-60 –Protons? –Electrons? –Mass number? –Neutrons?

Practice • Magnesium-26 –Protons? –Electrons? –Mass number? –Neutrons?

Practice P –Protons? –Electrons? –Mass number? –Neutrons? • 15 29
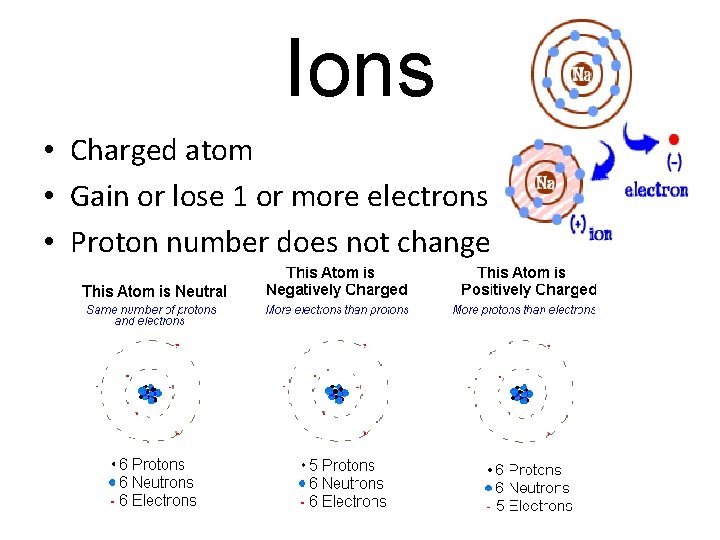
Ions • Charged atom • Gain or lose 1 or more electrons • Proton number does not change

Practice How many electrons in a neutral atom of Na? How many protons in Na? How many electrons in Na +1

Practice How many electrons in a neutral atom of S? How many protons in S? How many electrons in S -2?
- Slides: 25