Atomic Structure and the Periodic Table Warm Up
Atomic Structure and the Periodic Table
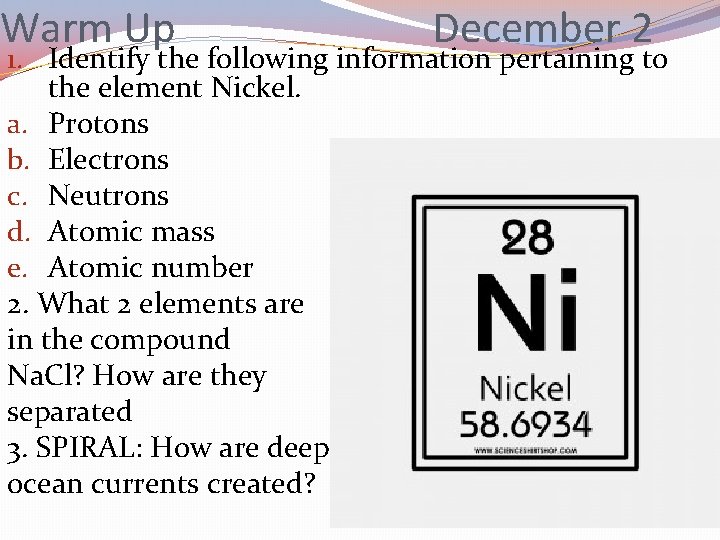
Warm Up December 2 1. Identify the following information pertaining to the element Nickel. a. Protons b. Electrons c. Neutrons d. Atomic mass e. Atomic number 2. What 2 elements are in the compound Na. Cl? How are they separated 3. SPIRAL: How are deep ocean currents created?

Warm Up December 2 1. Identify the following information pertaining to the element Chlorine. a. Protons b. Electrons c. Neutrons d. Atomic mass e. Atomic number 2. What 2 elements are in the compound Na. Cl? How are they separated 3. SPIRAL: How are deep ocean currents created?
Warm Up November 15 1. What is the difference between a heterogeneous mixture and a homogeneous solution? 2. Determine whether the samples below are a mixture or a solution: -sand water -salt water -blue Kool-Aid -pepper and water Objectives: 8. P. 1. 2 -TSW understand how elements are organized on the periodic table. Essential Question: -How are elements arranged on the periodic table?
Warm Up November 16 1. What are the characteristics of a metal? 2. What are the characteristics of a nonmetal? Objectives: 8. P. 1. 1/1. 2 -TSE understand the atomic structure. -TSW understand how elements are arranged on the periodic table. Essential Questions: -How are elements arranged on the periodic table?
Warm Up November 21 How are the elements on the periodic table organized? be as specific as possible… 2. How does the new periodic table of elements differ from the periodic table Mendeleev created in the 1800’s? 3. Identify whether the following substances are basic elements, molecules, or compounds? H 2 O CO 2 Na. Cl O 1. Objectives: 8. P. 1. 1/8. P. 1. 2 -TSW understand the arrangement of the periodic table including group properties and individual uses. Essential Questions: -How are elements placed into groups and periods on the periodic table?

Atoms are the Smallest Form of Elements �All matter (liquids, solids, and gasses) is made up of atoms. �Atoms are the tiniest building blocks of all matter. �Atoms of the element hydrogen account for 90% of the total mass of the universe. �Element- consists of only one type of atom �Each element has its own unique symbol �H= hydrogen, S= sulfur, Au= gold, Pb= lead �All elements are made up of different atoms.
The Structure of an Atom �Although atoms are the tiniest building blocks of all matter they are made up of even smaller particles �Atoms are composed of three types of particles �Electrons, protons, and neutron �Proton – a positively charged particle located in the atoms nucleus �Neutron – A particle that has no electric charge located in an atom’s nucleus. �Electron – A negatively charged particle located outside an atom’s nucleus.
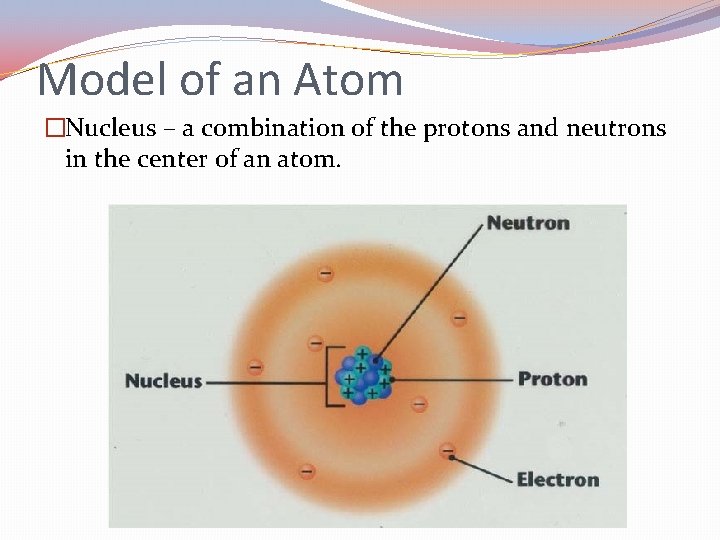
Model of an Atom �Nucleus – a combination of the protons and neutrons in the center of an atom.
More about Atoms �Atoms are small. So small you can fit millions of atoms in the period at the end of this sentence. �Electrons are 2000 times smaller than protons and neutrons. �The type of atom an element has is determined by the number of protons called the atomic number. �Every gold atom (Au) has 79 protons. What is its atomic number ____? Hydrogen?
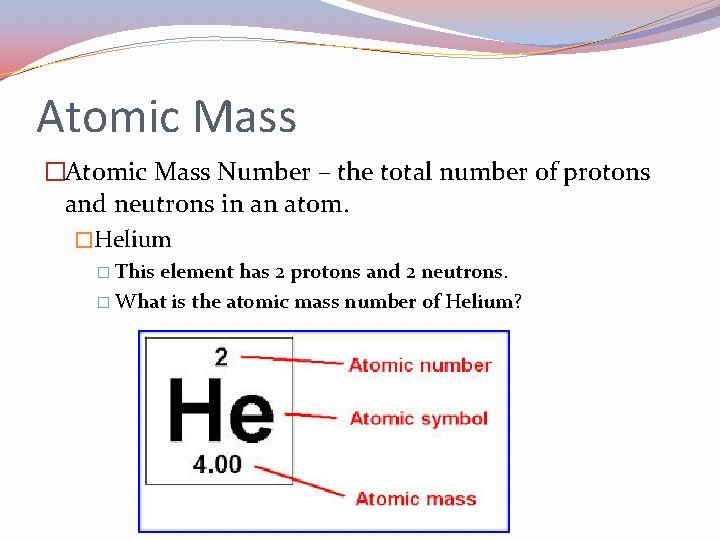
Atomic Mass �Atomic Mass Number – the total number of protons and neutrons in an atom. �Helium � This element has 2 protons and 2 neutrons. � What is the atomic mass number of Helium?
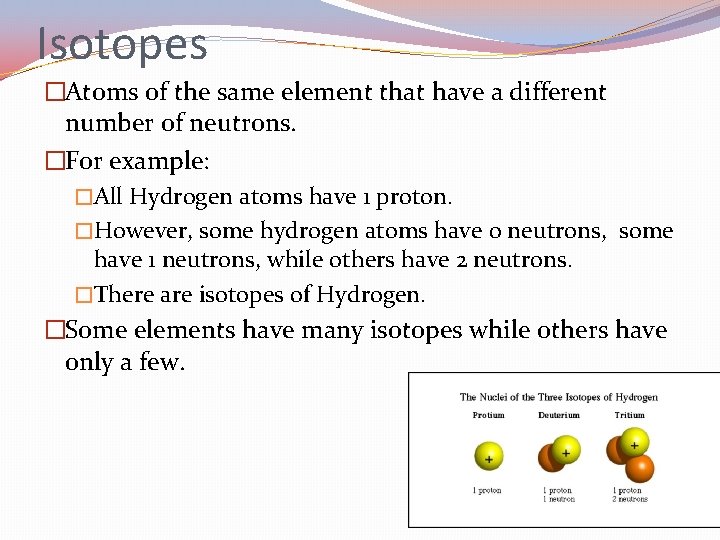
Isotopes �Atoms of the same element that have a different number of neutrons. �For example: �All Hydrogen atoms have 1 proton. �However, some hydrogen atoms have 0 neutrons, some have 1 neutrons, while others have 2 neutrons. �There are isotopes of Hydrogen. �Some elements have many isotopes while others have only a few.
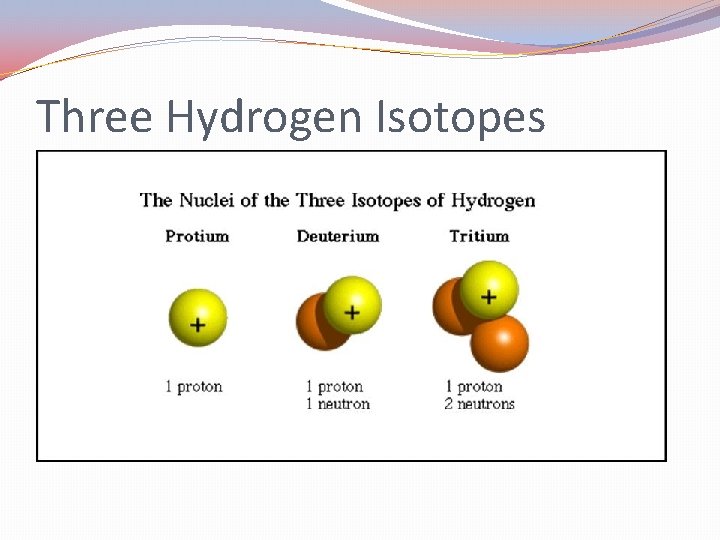
Three Hydrogen Isotopes

Ion �An atom or a group of atoms that have a positive or negative electric charge. �An ion is formed when an atom loses or gains one or more electrons. When an atom is attracted to another atom because it has an unequal number of electrons and protons, the atom is called an ION.

Positive Ions vs. Negative Ions POSITIVE Ions Negative Ions �Positive Ions form when an atom loses 1 or more electrons. �Atoms with more protons than electrons will have a positive charge �H + Li + Na + K + �Negative Ions form when an atom loses 1 or more protons. �Atoms with more electrons than protons will have a negative charge. �F - Cl - Br - I – � Hydrogen, Lithium, Nickel, Potassium � Fluoride, Chlorine, Bromine, Iodine

1 D Reflection Questions 1. What is the most common element in the universe? In your body? 2. What are the major parts of an atom? 3. Which part of an atom has a negative charge? 4. How is the atomic mass number different from the atomic number? 5. What must happen to form an ion?

Discussion Points 1 1. How is the modern periodic table organized? 2. What is the + charged particle of an atom known as? 3. What is the negatively charged particle of an atom known as? 4. These elements on the periodic table contain properties of both metals and nonmetals. 5. How did Mendeleev organize the periodic table?
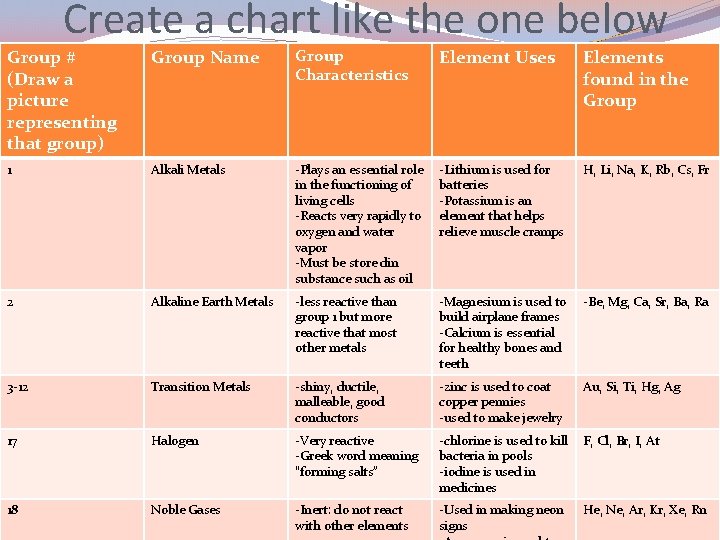
Create a chart like the one below Group # (Draw a picture representing that group) Group Name Group Characteristics Element Uses Elements found in the Group 1 Alkali Metals -Plays an essential role in the functioning of living cells -Reacts very rapidly to oxygen and water vapor -Must be store din substance such as oil -Lithium is used for batteries -Potassium is an element that helps relieve muscle cramps H, Li, Na, K, Rb, Cs, Fr 2 Alkaline Earth Metals -less reactive than group 1 but more reactive that most other metals -Magnesium is used to build airplane frames -Calcium is essential for healthy bones and teeth -Be, Mg, Ca, Sr, Ba, Ra 3 -12 Transition Metals -shiny, ductile, malleable, good conductors -zinc is used to coat copper pennies -used to make jewelry Au, Si, Ti, Hg, Ag 17 Halogen -Very reactive -Greek word meaning “forming salts” -chlorine is used to kill bacteria in pools -iodine is used in medicines F, Cl, Br, I, At 18 Noble Gases -Inert: do not react with other elements -Used in making neon signs He, Ne, Ar, Kr, Xe, Rn
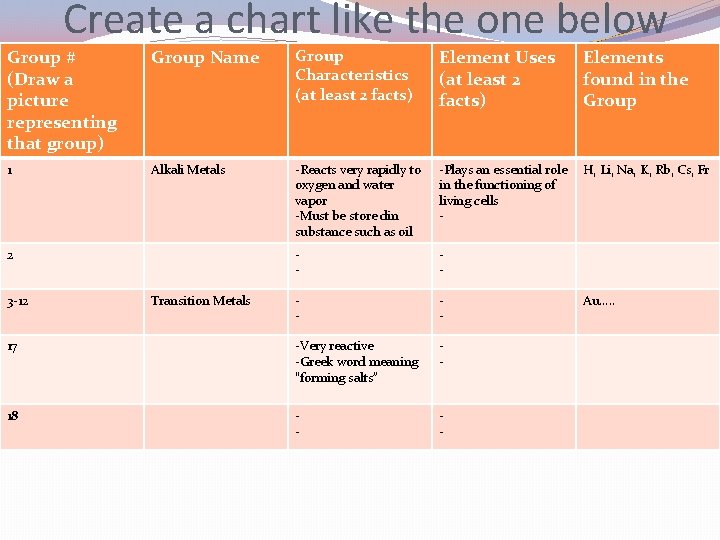
Create a chart like the one below Group # (Draw a picture representing that group) Group Name Group Characteristics (at least 2 facts) Element Uses (at least 2 facts) Elements found in the Group 1 Alkali Metals -Reacts very rapidly to oxygen and water vapor -Must be store din substance such as oil -Plays an essential role in the functioning of living cells - H, Li, Na, K, Rb, Cs, Fr - - 17 -Very reactive -Greek word meaning “forming salts” - 18 - - 2 3 -12 Transition Metals Au…. .

Discussion Points 2 1. 2. 3. 4. 5. Which group(s) on the periodic table are most reactive? Which group(s) on the periodic table are the least reactive? Identify the names of the following groups: group 1, group 2, group 3 -12, group 17, and group 18 What are the major characteristics of metals? What are two uses for elements found in group 17?
Greatest Discoveries: Chemical Structures �Pre-observing question: �What chemical reaction have you observed? �Post observing questions: �How did August Kekule’s discovery about carbon change the science of chemistry? �Why is the periodic table of elements so important to science? �Why did Mendeleev create one of the first periodic table of elements?
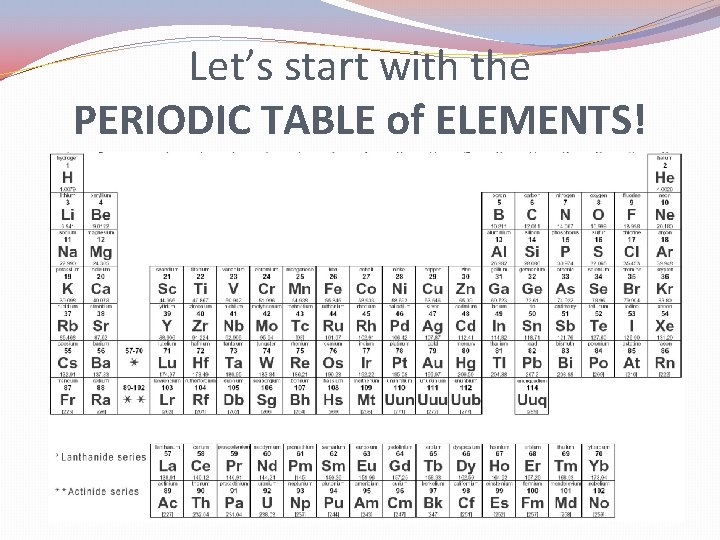
Let’s start with the PERIODIC TABLE of ELEMENTS!

Arrangement of the Periodic Table Activity 1 �You will receive 25 cards. �You will receive 5 different colors �Each card will be labels the following: SUV, Van, Truck, 4 -door, and 2 -door
Page 21 D Elements- How would you arrange these elements on the periodic table? H-hydrogen Li-lithium Na-sodium K- potassium He- Helium Be- beryllium Mg- magnesium Ca-calcium B- boron Ne- neon C-carbon N- nitrogen O- oxygen F- fluorine Cl- chlorine

The PERIODIC TABLE of ELEMENTS are the building blocks of EVERYTHING in our UNIVERSE!
QUESTION 1: What’s a four-letter word for ELEMENT.

Remember, the PERIODIC TABLE of ELEMENTS are the building blocks of EVERYTHING in our UNIVERSE! �Including all matter such as solids, liquids, and gas… �That includes you and me!
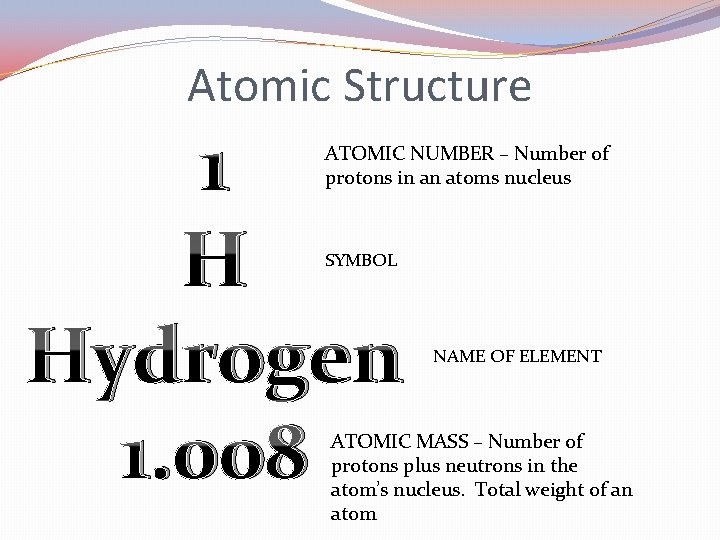
Atomic Structure 1 H Hydrogen 1. 008 ATOMIC NUMBER – Number of protons in an atoms nucleus SYMBOL NAME OF ELEMENT ATOMIC MASS – Number of protons plus neutrons in the atom’s nucleus. Total weight of an atom

Periodic Table Activity Procedures Directions: Using your textbook pages 21 D-22 D complete the activity below. 1. Obtain a copy of the periodic table. 2. Label the groups (columns) 1 -18 at the top of each column. 3. Label the periods (rows) 1 -7 on the left side of your periodic table. 4. Using a dark color, draw a zig zag line separating the metals from non metals. This is called the stair case line. 5. Lightly color the metals yellow, non metals green, and metalloids purple. 6. Define the following terms (6) on the front of your periodic table: nonmetals, metalloids, groups, periods, and stair case line. Provide at least on example of
Q 3: Elements are organized by periods and groups. Write down which one goes left to right, & which one goes up & down.
Q 3: Elements are organized by periods and groups. Write down which one goes left to right, & which one goes up & down.
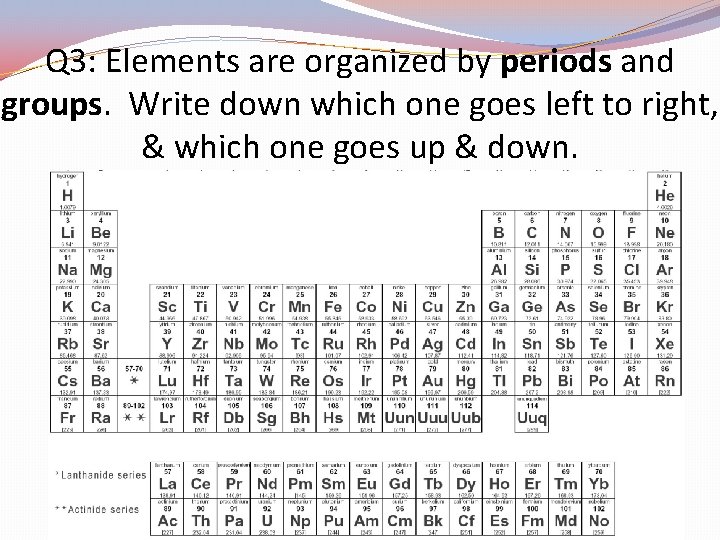
Q 3: Elements are organized by periods and groups. Write down which one goes left to right, & which one goes up & down.
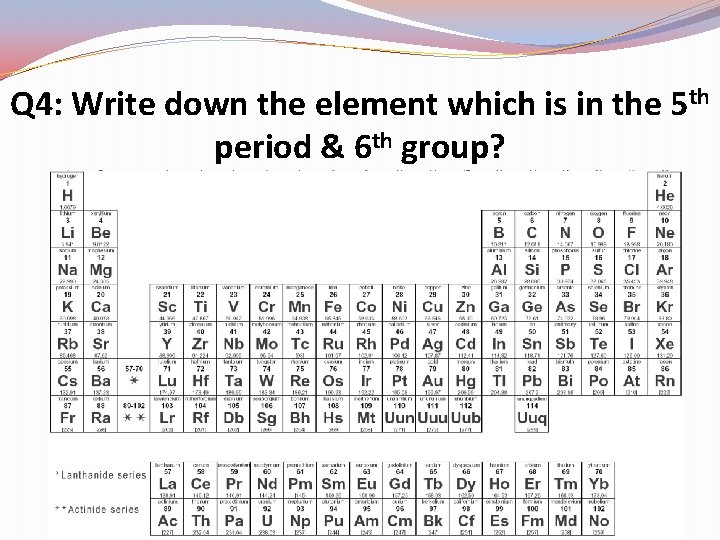
Q 4: Write down the element which is in the 5 th period & 6 th group?
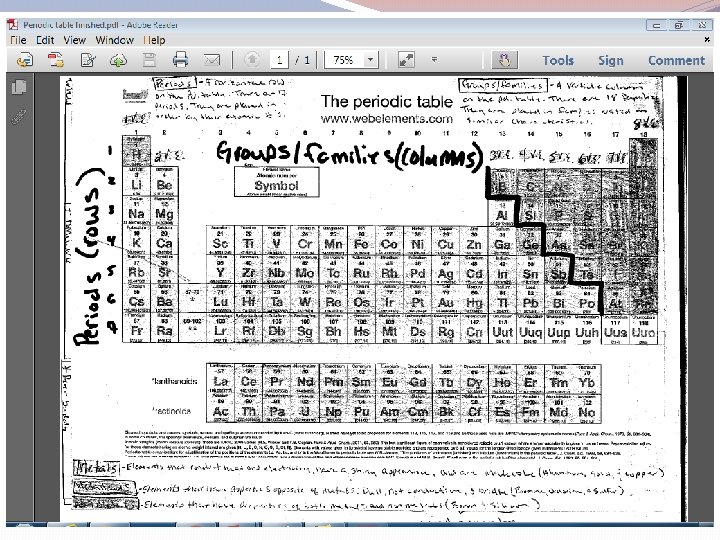
The Periodic Table �The modern periodic table is organized based on two factors: 1. Characteristics – elements are placed in groups or families based on their common characteristics. 2. Atomic number – elements are also placed in rows or periods based on their atomic number. Discussion Point: what do you think happens to the density of the atoms as you move from left to right on the periodic table?
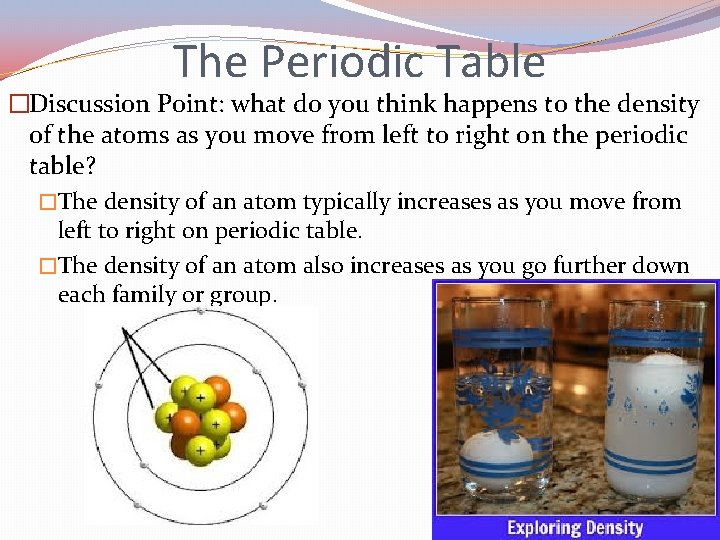
The Periodic Table �Discussion Point: what do you think happens to the density of the atoms as you move from left to right on the periodic table? �The density of an atom typically increases as you move from left to right on periodic table. �The density of an atom also increases as you go further down each family or group.
Q 5: Elements that are in the same _____ can act and behave similar.

What does the (+) part of an atom represent?

What does the (+) part of an atom represent? What does the (-) part of an atom represent? What does the (n) part of an atom represent?
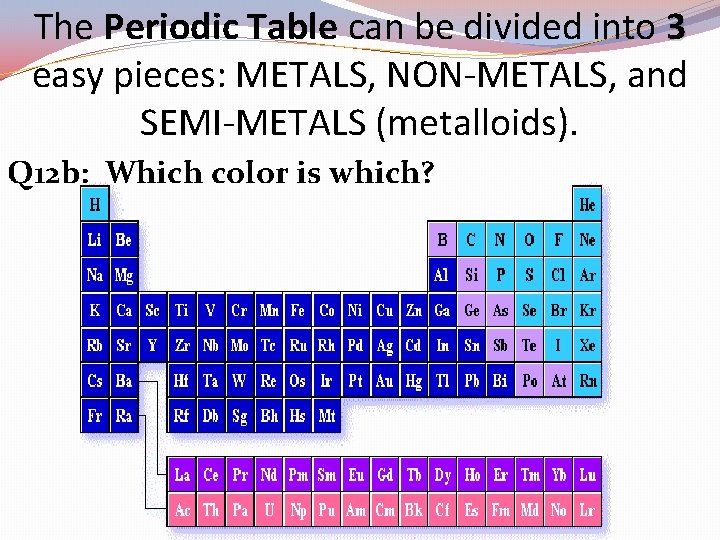
The Periodic Table can be divided into 3 easy pieces: METALS, NON-METALS, and SEMI-METALS (metalloids). Q 12 b: Which color is which?
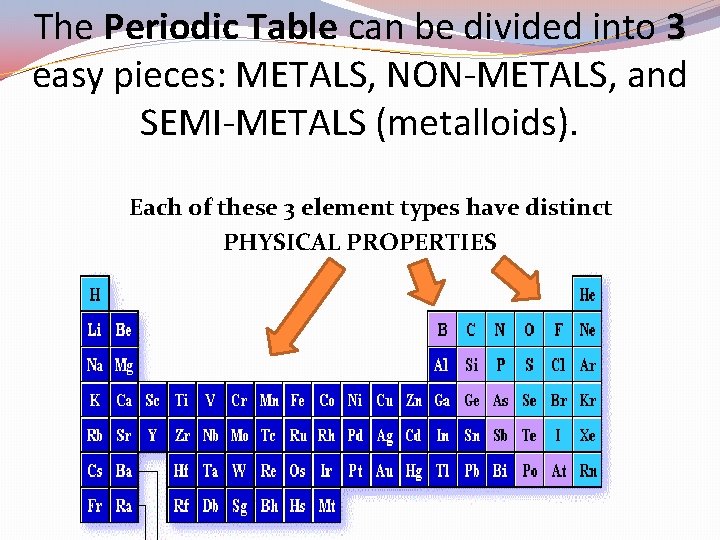
The Periodic Table can be divided into 3 easy pieces: METALS, NON-METALS, and SEMI-METALS (metalloids). Each of these 3 element types have distinct PHYSICAL PROPERTIES
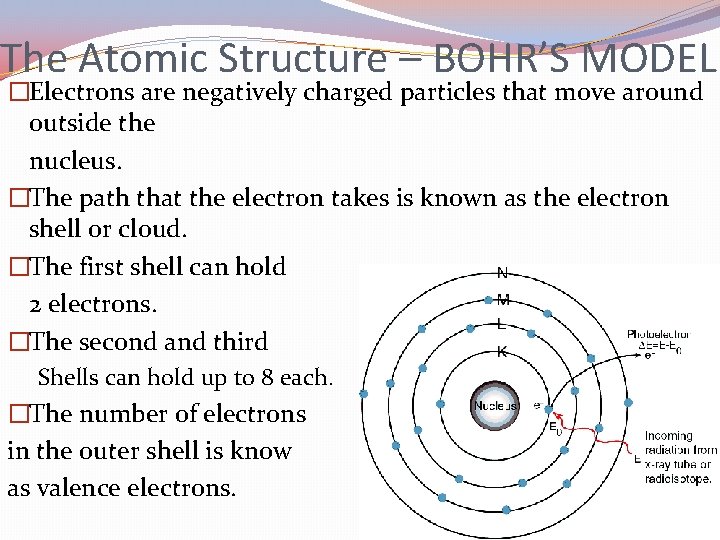
The Atomic Structure – BOHR’S MODEL �Electrons are negatively charged particles that move around outside the nucleus. �The path that the electron takes is known as the electron shell or cloud. �The first shell can hold 2 electrons. �The second and third Shells can hold up to 8 each. �The number of electrons in the outer shell is know as valence electrons.
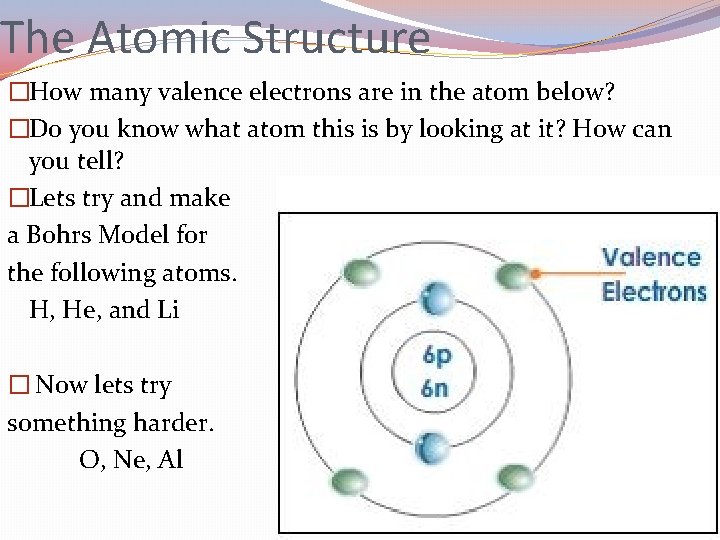
The Atomic Structure �How many valence electrons are in the atom below? �Do you know what atom this is by looking at it? How can you tell? �Lets try and make a Bohrs Model for the following atoms. H, He, and Li � Now lets try something harder. O, Ne, Al
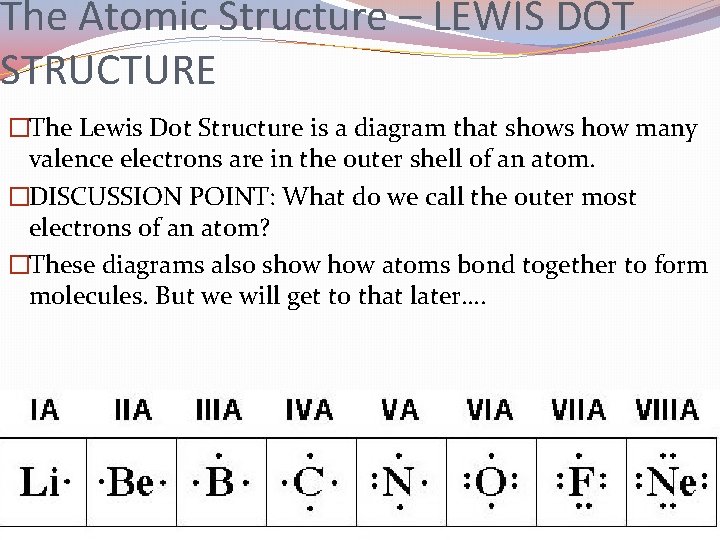
The Atomic Structure – LEWIS DOT STRUCTURE �The Lewis Dot Structure is a diagram that shows how many valence electrons are in the outer shell of an atom. �DISCUSSION POINT: What do we call the outer most electrons of an atom? �These diagrams also show atoms bond together to form molecules. But we will get to that later….
The Atomic Structure – LEWIS DOT STRUCTURE �Lets revisit our Bohr’s Model to create a Lewis Dot Structure for each atom. H He �Let’s try the harder ones: O Ne Li Al

Physical properties are the physical ways we can describe a substance.

Physical properties are the physical ways we can describe a substance. BOILING POINT

Physical properties are the physical ways we can describe a substance. FREEZING POINT

Physical properties are the physical ways we can describe a substance. MALLEABLE

Physical properties are the physical ways we can describe a substance. Ductile – easily drawn or pulled into thin wires. Malleable – Easily flattened and shaped

Physical properties are the physical ways we can describe a substance. solubility

Physical properties are the physical ways we can describe a substance. density

All & more can be used to physically describe something. aka: physical properties
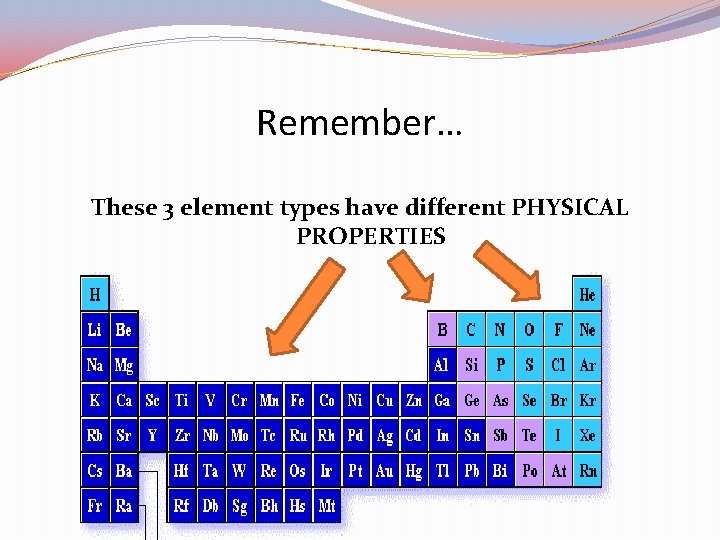
Remember… These 3 element types have different PHYSICAL PROPERTIES

Q 13: Identify whether it’s METALS or NON-METALS that generally share the following physical properties. Write these physical properties: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Shiny Less Dense Mostly gas at room temp. Conducts heat Malleable Low boiling point Conducts electricity High melting point Ductile Found in all 3 states (s, l, g) Choose: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Metals or Non-metals Metals or Non-metals Metals or Non-metals

Q 24: Write the definition of MOLECULE?

Q 25: Write down the one that is a molecule? 1. 2. 3. 4. N Ni NO No Explain your answer in one sentence.

All compounds are molecules, but not all molecules are compounds.

Q 25 b: Which molecule below is NOT a compound? Here’s your list of molecules again. 1. 2. 3. 4. 5. 6. CO KCl H 2 O C 6 H 12 O 6 Na. I N 2 Explain your answer in one sentence.

SO, MOST MOLECULES HAVE SUBSCRIPTS.

SO, MOST MOLECULES HAVE SUBSCRIPTS. SUB because the number goes BELOW.

SO, MOST MOLECULES HAVE SUBSCRIPTS. SUB because the number goes BELOW. Like a SUBmarine!

SUBSCRIPTS tell you if there are MORE than 1 ATOM of that ELEMENT in that particular MOLECULE. Q 26: Write how many hydrogen atoms are in 1 water molecule? Q 27: Write how many oxygen atoms are in a single water molecule?
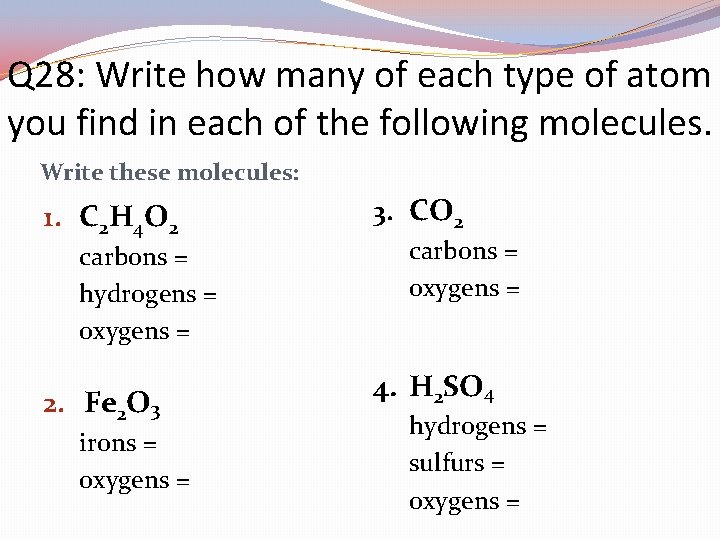
Q 28: Write how many of each type of atom you find in each of the following molecules. Write these molecules: 1. C 2 H 4 O 2 carbons = hydrogens = oxygens = 2. Fe 2 O 3 irons = oxygens = 3. CO 2 carbons = oxygens = 4. H 2 SO 4 hydrogens = sulfurs = oxygens =

We only have 100 + elements in our universe… …but these elements can combine in almost an INFINITE number of molecules.
Our bodies, for instance, are made of about 7, 000, 000, 000 atoms. �Because we are 70% water, and water is 2: 1 hydrogen to oxygen…
Our bodies, for instance, are made of about 7, 000, 000, 000 atoms. �Because we are 70% water, and water is 2: 1 hydrogen to oxygen… �…and because hydrogen is very common in other molecular compounds in our body…
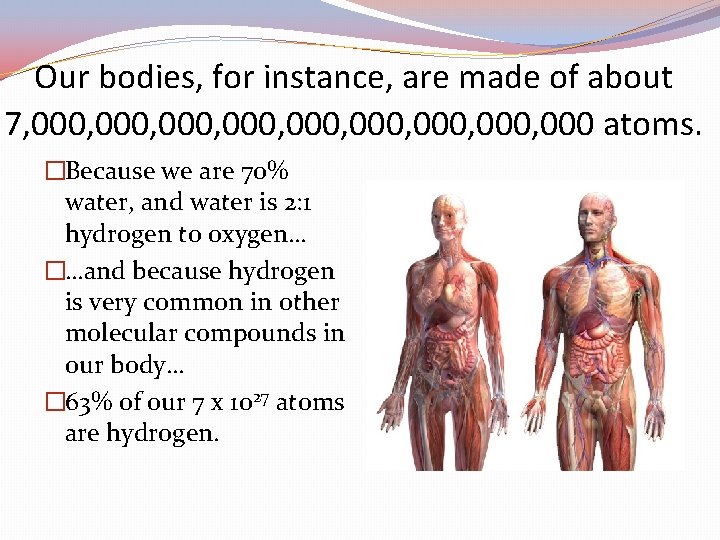
Our bodies, for instance, are made of about 7, 000, 000, 000 atoms. �Because we are 70% water, and water is 2: 1 hydrogen to oxygen… �…and because hydrogen is very common in other molecular compounds in our body… � 63% of our 7 x 1027 atoms are hydrogen.

63% of our body’s atoms are hydrogen, but only 10% of our weight is hydrogen. Q 29: Write down why you think this is so.

Our bodies are made of about 7, 000, 000, 000 atoms. � 63% of our atoms are hydrogens, 10% of our weight. � 24% of our atoms are oxygen atoms, 65% of our weight. � 12% of our atoms are carbon atoms, 18% of our weight.

Q 30: If so much of us is oxygen and hydrogen atoms, why aren’t we mostly made of gas?

New stuff is made by CHEMICAL REACTIONS!

Chemical reactions occur when two or more substances combine to form something new.

Chemical reactions occur when two or more substances combine to form something new.

Chemical reactions occur when two or more substances combine to form something new.

Chemical reactions occur when two or more substances combine to form something new.

Chemical reactions occur when two or more substances combine to form something new.

Chemical reactions occur when two or more substances combine to form something new. �Like when wax & oxygen fire up in a candle. . .

Chemical reactions occur when two or more substances combine to form something new. �Like when wax & oxygen fire up in a candle. . . �Like your classic baking soda & vinegar. . .

Chemical reactions mean a chemical change has taken place.

Q 31: Write down whether the following are examples of physical or chemical change.

New molecules are formed when a chemical reaction takes place.
New molecules are formed when a chemical reaction takes place. �Like when wax & oxygen fire up in a candle. . .
New molecules are formed when a chemical reaction takes place. �Like when wax & oxygen fire up in a candle. . . WAX + OXYGEN CARBON DIOXIDE + WATER
New molecules are formed when a chemical reaction takes place. �Like when wax & oxygen fire up in a candle. . . WAX + OXYGEN CARBON DIOXIDE + WATER + HEAT

New molecules are formed when a chemical reaction takes place. �Like when wax & oxygen fire up in a candle. . . WAX + OXYGEN CARBON DIOXIDE + WATER + HEAT �Like your classic baking soda & vinegar. . .

New molecules are formed when a chemical reaction takes place. �Like when wax & oxygen fire up in a candle. . . WAX + OXYGEN CARBON DIOXIDE + WATER + HEAT �Like your classic baking soda & vinegar. . . BAKING SODA + VINEGAR CARBON DIOXIDE + WATER + SODIUM ACETATE

Q 32: Write down 4 ways you can tell if a chemical reaction (chemical change) has occurred.

In a chemical reaction, the INPUTS (ingredients) are called REACTANTS

The OUTPUTS are called PRODUCTS.
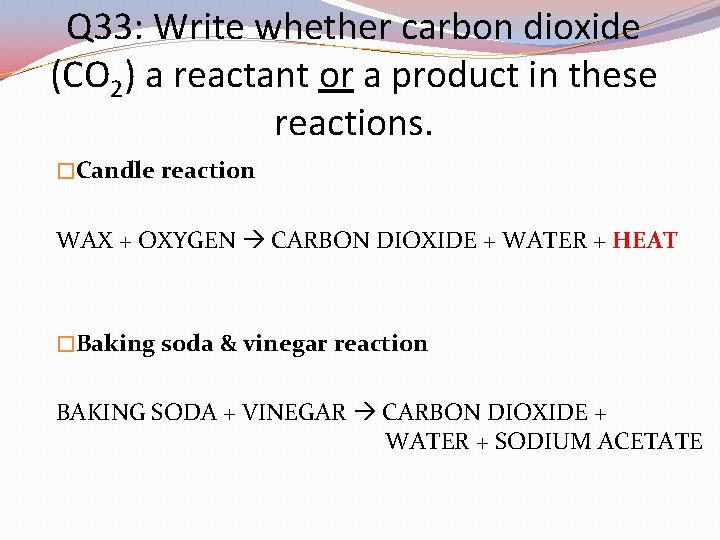
Q 33: Write whether carbon dioxide (CO 2) a reactant or a product in these reactions. �Candle reaction WAX + OXYGEN CARBON DIOXIDE + WATER + HEAT �Baking soda & vinegar reaction BAKING SODA + VINEGAR CARBON DIOXIDE + WATER + SODIUM ACETATE

Chemical reactions can be abbreviated (shortened) into chemical equations.

Chemical reactions can be abbreviated (shortened) into chemical equations.

Chemical reactions can be abbreviated (shortened) into chemical equations. �Candle reaction WAX + OXYGEN CARBON DIOXIDE + WATER + HEAT �Baking soda & vinegar reaction BAKING SODA + VINEGAR CARBON DIOXIDE + WATER + SODIUM ACETATE
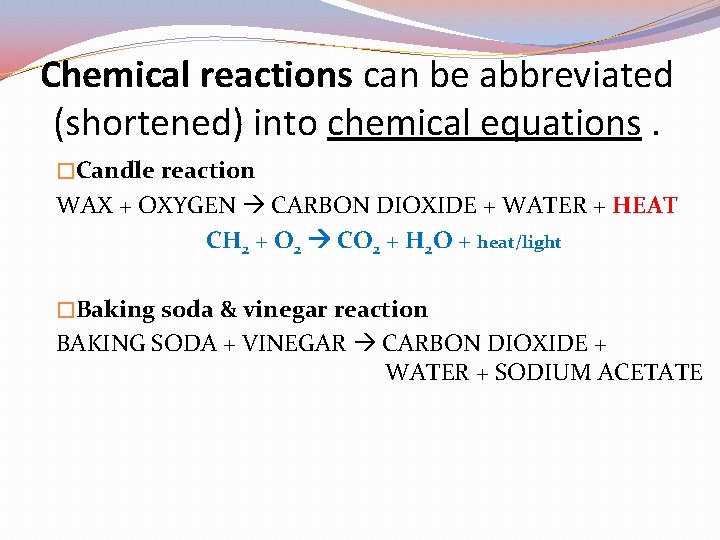
Chemical reactions can be abbreviated (shortened) into chemical equations. �Candle reaction WAX + OXYGEN CARBON DIOXIDE + WATER + HEAT CH 2 + O 2 CO 2 + H 2 O + heat/light �Baking soda & vinegar reaction BAKING SODA + VINEGAR CARBON DIOXIDE + WATER + SODIUM ACETATE
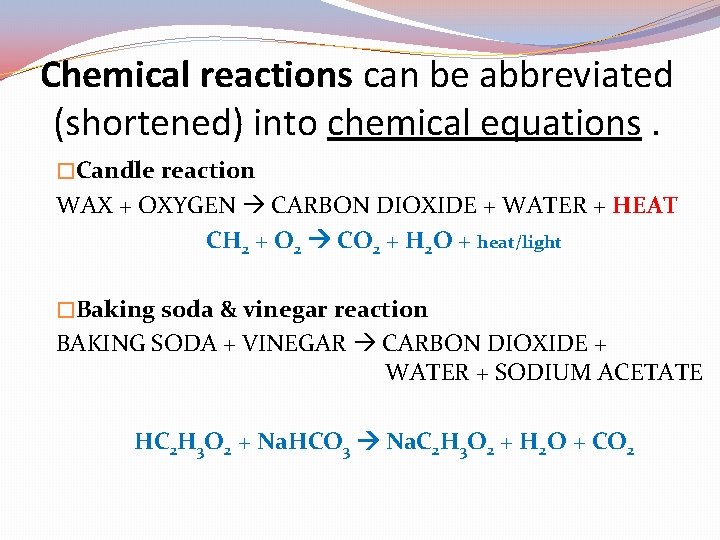
Chemical reactions can be abbreviated (shortened) into chemical equations. �Candle reaction WAX + OXYGEN CARBON DIOXIDE + WATER + HEAT CH 2 + O 2 CO 2 + H 2 O + heat/light �Baking soda & vinegar reaction BAKING SODA + VINEGAR CARBON DIOXIDE + WATER + SODIUM ACETATE HC 2 H 3 O 2 + Na. HCO 3 Na. C 2 H 3 O 2 + H 2 O + CO 2

Q 34: Write how many products are in a baking soda & vinegar reaction. �Candle reaction WAX + OXYGEN CARBON DIOXIDE + WATER + HEAT CH 2 + O 2 CO 2 + H 2 O + heat/light �Baking soda & vinegar reaction BAKING SODA + VINEGAR CARBON DIOXIDE + WATER + SODIUM ACETATE HC 2 H 3 O 2 + Na. HCO 3 Na. C 2 H 3 O 2 + H 2 O + CO 2
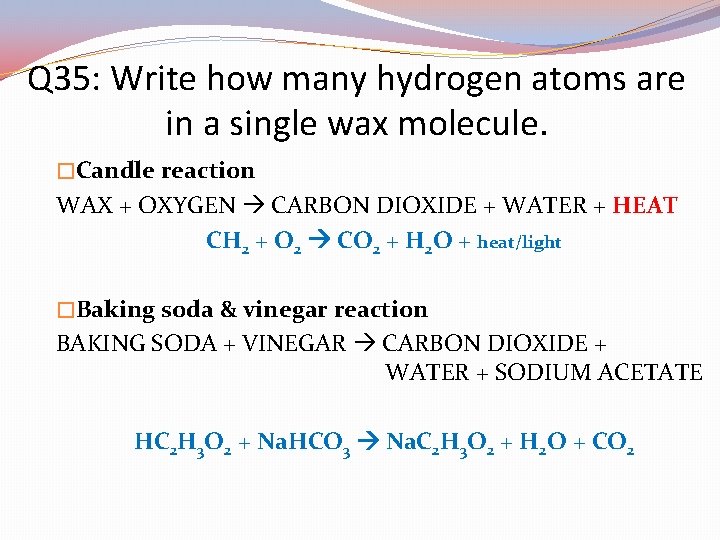
Q 35: Write how many hydrogen atoms are in a single wax molecule. �Candle reaction WAX + OXYGEN CARBON DIOXIDE + WATER + HEAT CH 2 + O 2 CO 2 + H 2 O + heat/light �Baking soda & vinegar reaction BAKING SODA + VINEGAR CARBON DIOXIDE + WATER + SODIUM ACETATE HC 2 H 3 O 2 + Na. HCO 3 Na. C 2 H 3 O 2 + H 2 O + CO 2
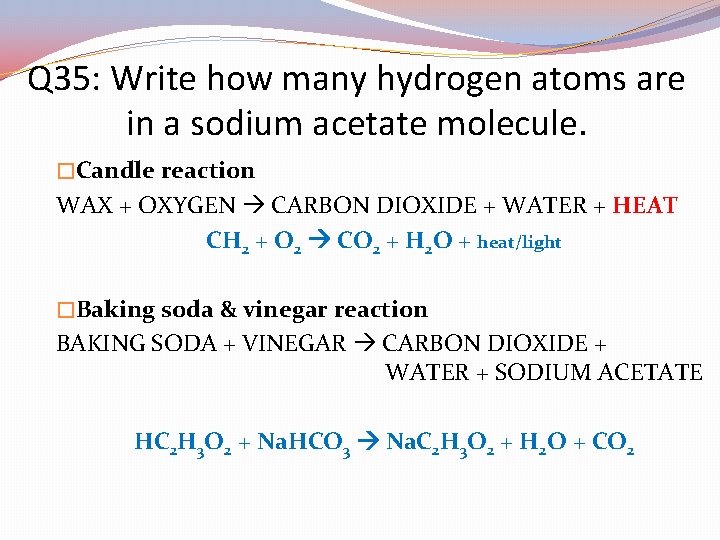
Q 35: Write how many hydrogen atoms are in a sodium acetate molecule. �Candle reaction WAX + OXYGEN CARBON DIOXIDE + WATER + HEAT CH 2 + O 2 CO 2 + H 2 O + heat/light �Baking soda & vinegar reaction BAKING SODA + VINEGAR CARBON DIOXIDE + WATER + SODIUM ACETATE HC 2 H 3 O 2 + Na. HCO 3 Na. C 2 H 3 O 2 + H 2 O + CO 2

Remember PHOTOSYNTHESIS?

Remember PHOTOSYNTHESIS? It’s how plants take sunlight, water & the air we exhale and turn it into sugar food for them and oxygen for us!
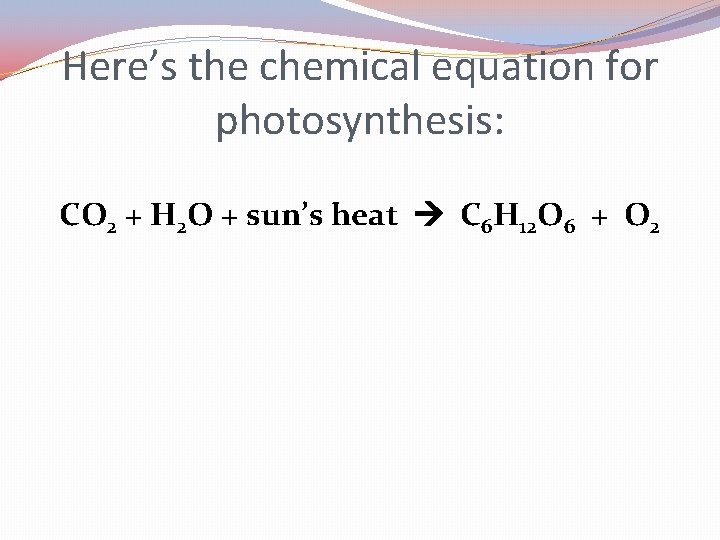
Here’s the chemical equation for photosynthesis: CO 2 + H 2 O + sun’s heat C 6 H 12 O 6 + O 2
Remember Cell Respiration?
Remember Cell Respiration? Mitochondria in our cells are able to take oxygen we breathe & sugar we eat and turn it into energy & waste products.
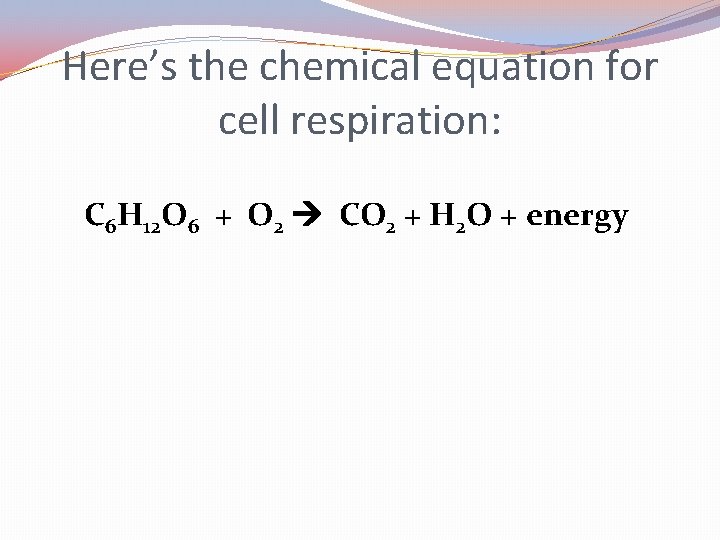
Here’s the chemical equation for cell respiration: C 6 H 12 O 6 + O 2 CO 2 + H 2 O + energy
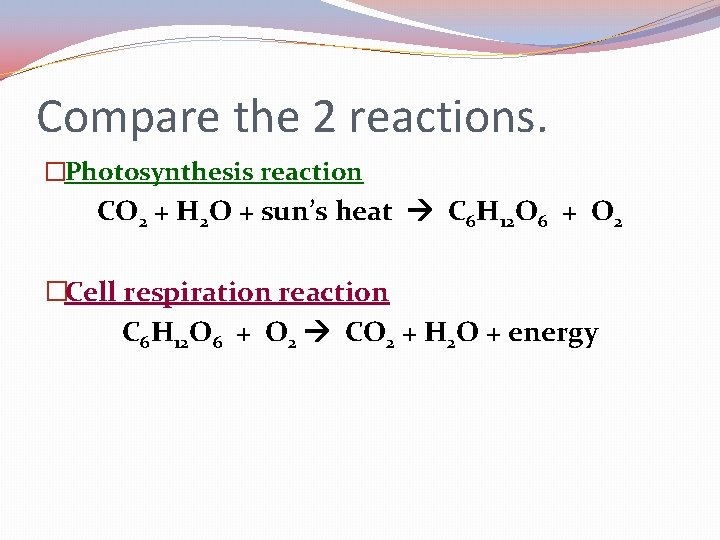
Compare the 2 reactions. �Photosynthesis reaction CO 2 + H 2 O + sun’s heat C 6 H 12 O 6 + O 2 �Cell respiration reaction C 6 H 12 O 6 + O 2 CO 2 + H 2 O + energy
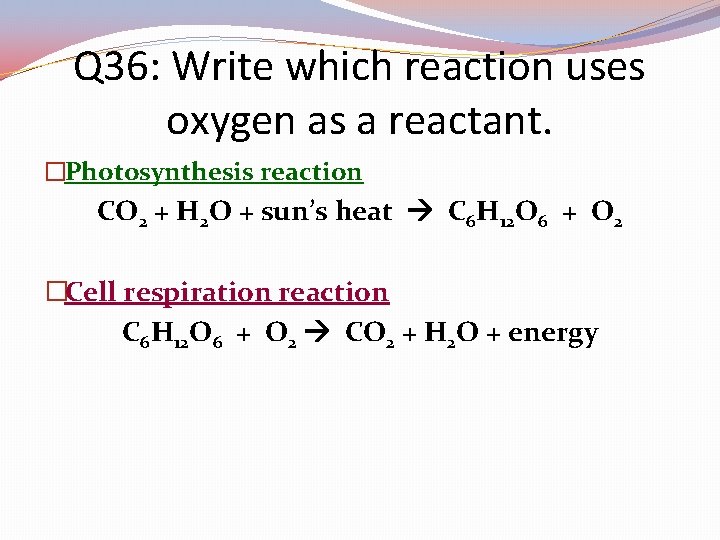
Q 36: Write which reaction uses oxygen as a reactant. �Photosynthesis reaction CO 2 + H 2 O + sun’s heat C 6 H 12 O 6 + O 2 �Cell respiration reaction C 6 H 12 O 6 + O 2 CO 2 + H 2 O + energy
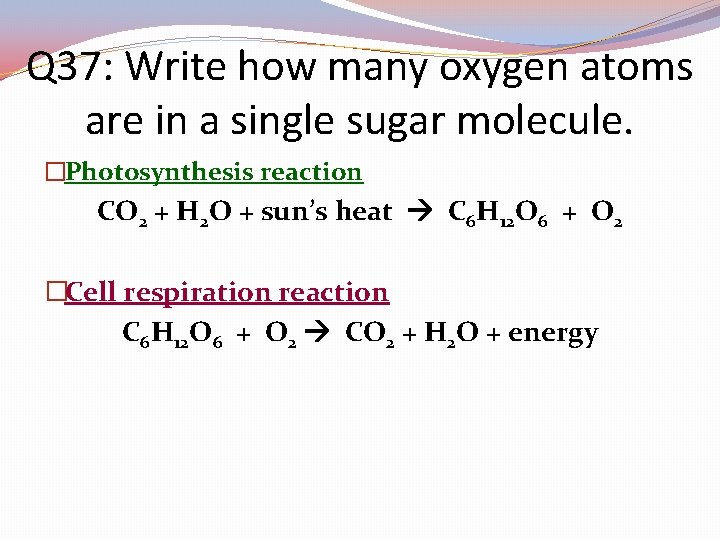
Q 37: Write how many oxygen atoms are in a single sugar molecule. �Photosynthesis reaction CO 2 + H 2 O + sun’s heat C 6 H 12 O 6 + O 2 �Cell respiration reaction C 6 H 12 O 6 + O 2 CO 2 + H 2 O + energy
Q 38: Write the chemical formula for oxygen gas. �Photosynthesis reaction CO 2 + H 2 O + sun’s heat C 6 H 12 O 6 + O 2 �Cell respiration reaction C 6 H 12 O 6 + O 2 CO 2 + H 2 O + energy
Q 39: Write whether oxygen gas is a molecule, a compound, or both. �Photosynthesis reaction CO 2 + H 2 O + sun’s heat C 6 H 12 O 6 + O 2 �Cell respiration reaction C 6 H 12 O 6 + O 2 CO 2 + H 2 O + energy

Q 40: Write whether the candle wax reaction is endothermic or exothermic

But WAIT there’s MORE!

You can’t just throw together all reactants & expect perfect products

You can’t just throw together all reactants & expect perfect products You’ll get bad pancakes.

IN CHEMISTRY, EVERYTHING MUST BE MEASURED & BALANCED!

Q 41: Fill in the following blanks. 1. C + O 2 CO 2 5 g +___ 18 g 2. CO 2 + Ca(OH)2 Ca. CO 3 + H 2 O 15 g + 100 g ______ + 50 g 3. Fe. O + CO Fe + CO 2 ____ + 6 g 8 g + 4 g 4. Ca. Cl 2 + Na 2 CO 3 2 Na. Cl + Ca. CO 3 12 mg+48 mg 40 mg+ _____

Even though different molecules are produced in a chemical reaction, the weights of the reactants must equal the weight of the products!

Even though different molecules are produced in a chemical reaction, the weights of the reactants must equal the weight of the products! total mass of reactants = total mass of products
The same goes for the total number of atoms in reactants versus products.
- Slides: 119