Atomic Structure and The Periodic Table Mr Chan

Atomic Structure and The Periodic Table Mr. Chan Northwestern University

Today (Day 18) n n n (0 -10) Return/Discuss Tests (10 -15) Activate: Sketch Atom (15 -20) Mystery Box (20 -30) Dalton’s Atomic Theory (30 -43) Word Splash Video – WOC The Atom (30 min) n Conferences

Mystery Boxes n n Procedure: Select a Mystery Box. Without opening it, try to learn about its contents by using all the tools of observation available to you. Outline your plan to determine the contents of the box and record all observations you make.

Mystery Boxes n n Questions: 1. What evidence supports your conclusion about the type of item contained in the box? 2. What kinds of things can you do to determine the contents of your box if you had access to other kinds of observational tools? 3. Since you cannot see what is inside, how can you be 100% sure of its contents?

What are atoms? n n n Smallest particle of element that retains properties of the element Possible to see individual atoms Demo: Al Foil – Physical Changes – Smallest Piece

History of Atomic Theory n n Original model – Democritus n Atomos – smallest unit of matter Dalton’s Theory n 1) Elements composed of tiny indivisible (not invisible) particles called atoms n 2) Atoms of the same element are identical n 3) Atoms of different elements can chemically combine with one another in simple whole number ratios (compounds) n 4) In chemical reactions, atoms are separated, joined, and rearranged. Atoms of one element are never changed.

Today (Day 19) n n n (0 -5) Discuss HW (5 -10) Demo (10 -25) Experiments – discovery of Atomic Structure (25 -35) Rutherford’s Au Experiment (35 -43, 0 -25) LAB (25 -43) Numbers associated with At. Structure

What is the structure of an atom? n Electrons n J. J. Thomson, cathode ray tubes – Overhead n Electric current flowing through gases n Beam – cathode ray travels from cathode (-) to the anode (+) n Metal plates – beam attracted to positive plate n Electrons: negative, 1/2000 mass of hydrogen atom n Millikan – oil drop experiments n determined charge/mass ratio of electron n Mass of electron = 1/1840 mass of hydrogen

Structure cont. n n Protons – positively charged particles n Needed to balance charge of electrons in atom n Goldstein – CRT with loss of electrons – canal rays from cathode traveling in opposite direction as electrons Neutrons – no charge, mass - proton n Chadwick – knew some mass was unaccounted for

Summary of Subatomic particles n Particle, symbol, relative charge, relative mass, actual mass
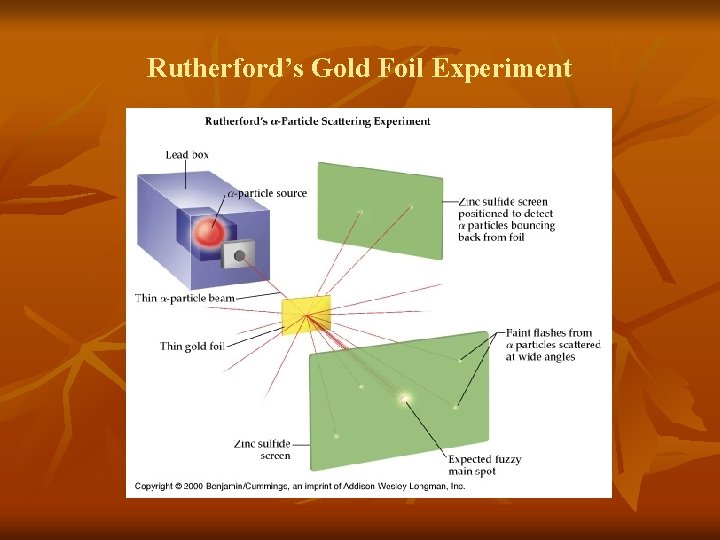
Rutherford’s Gold Foil Experiment

Rutherford’s Gold Foil Experiment n n n Led to discovery that most of the atom is empty space All of the positive charge and most of the atom’s mass found in a small region Nucleus of the atom n Core that contains protons and neutrons
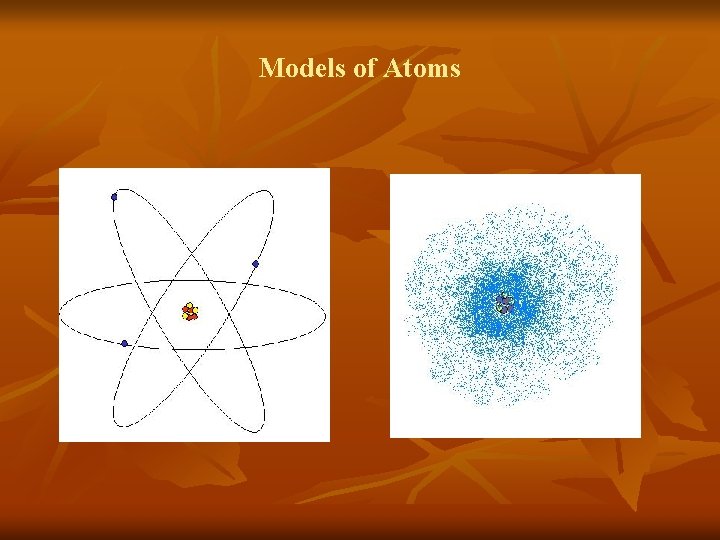
Models of Atoms

Numbers Associated with Atomic Structure n n Atomic Number n Number of protons in nucleus of atom of element n Also equals number of electrons if electrically neutral n Determines which element you have n Practice: How many protons and electrons, what the symbol, element name is Mass number n Total number of protons and neutrons n Not given on the periodic table
Calculating Number of Protons, Neutrons, and Electrons n n Number of neutrons = Mass Number – Atomic Number Shorthand Notation: Mass/Atomic – Element OR Element – Mass number Practice #9 -12: Determine number of protons, electrons, and neutrons, completing tables of atomic number, mass number, #protons, #neutrons, #electrons, symbol – notation, Determining from symbol: #neutrons

Today (Day 20) n n (0 -5) Opener (5 -15) Discuss HW (15 -30) Quiz (30 -43) Isotopes

What are Isotopes? n n Atoms that have the same number of protons but different numbers of neutrons Different mass numbers Atoms are chemically alike – behave similarly n Common misconception: radically different – NO! Examples: 3 isotopes of hydrogen n Tritium – Spiderman 2 errors – MOVIE CLIP n Gas, not solid

Today (Day 21) n n n (0 -10) Opener/Discuss HW (10 -15) Discuss QUIZ (15 -20) Spiderman 2 Clip (20 -30) Activity: Isotope Numbers Relay Race (30 -43) Average Atomic Mass (0 -43) Isotopes and Atomic Mass Lab

Opener - 9/29 n Determine the number of protons, neutrons, and electrons in the following isotopes: n Hydrogen-1, Hydrogen-2, and Hydrogen-3

What is average atomic mass? n n n n Weighted grades analogy Masses of subatomic particles ridiculously small – needed something more convenient Arbitrary amount set: Carbon-12 atom = 12. 00000 amu 1 amu (atomic mass unit) = 1/12 mass of C-12 atom Mass of a single proton/neutron – 1 amu Why are the masses decimals? n Most elements exist as a mixture of 2 or more isotopes Look at average atomic masses of elements – can you speculate which isotope is most common?

How do you calculate average atomic mass? n Average atomic mass = weighted average mass of atoms in naturally occurring sample Number on Periodic Table – explains why we could not use as mass number n Reflects both the mass and the n

Today (Day 22) Read Article n Socratic Seminar n

Today (Day 24) n Review

Today (Day 25) n Chapter 4 Test

Origin of Periodic Table Mendeleev n. Listed elements in columns and in order of increasing atomic mass n. Elements in groups with similar properties n Moseley n

Organization of Periodic Table Increasing atomic number n Periods n. Horizontal rows n Groups n. Vertical columns of elements n Periodic law n. Repetition of chemical and n
- Slides: 26