Atomic Structure and the Alpha Scattering Experiment John






- Slides: 15

Atomic Structure and the Alpha Scattering Experiment

John Dalton (1766 – 1844) John Dalton described atoms as tiny balls of material. He said the atoms of a particular element are all identical. These are original Dalton model atoms He used small wooden balls to model atoms and showed how they could combine to produce compounds

JJ Thompson (1856 – 1940) In 1897 at Cambridge University JJ Thomson discovered ‘Cathode rays’ On closer examination it was decided that these ‘rays’ were in fact tiny, negatively charged particles being emitted from atoms. He has discovered the electron! Suddenly it was realised that atoms were not the smallest particles that could exist. ‘Plum Pudding’ model Thomson came up with a model for atoms based on his discovery. He said that because atoms themselves had no charge there must be a positive charge inside the atoms to cancel out the charge on the negative electrons. He said that atoms were solid lumps of positively charge material with the electrons scattered through it like the fruit in a plum pudding.

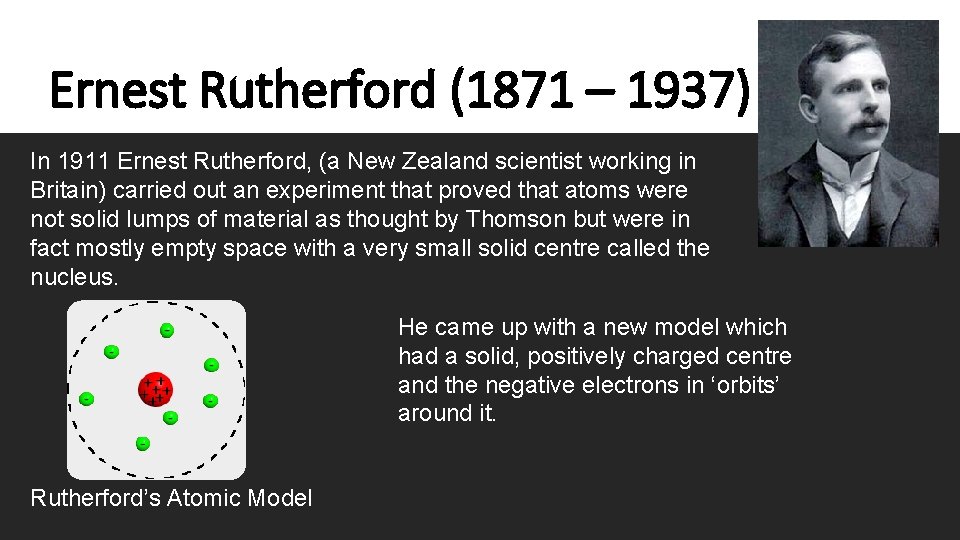
Ernest Rutherford (1871 – 1937) In 1911 Ernest Rutherford, (a New Zealand scientist working in Britain) carried out an experiment that proved that atoms were not solid lumps of material as thought by Thomson but were in fact mostly empty space with a very small solid centre called the nucleus. He came up with a new model which had a solid, positively charged centre and the negative electrons in ‘orbits’ around it. Rutherford’s Atomic Model

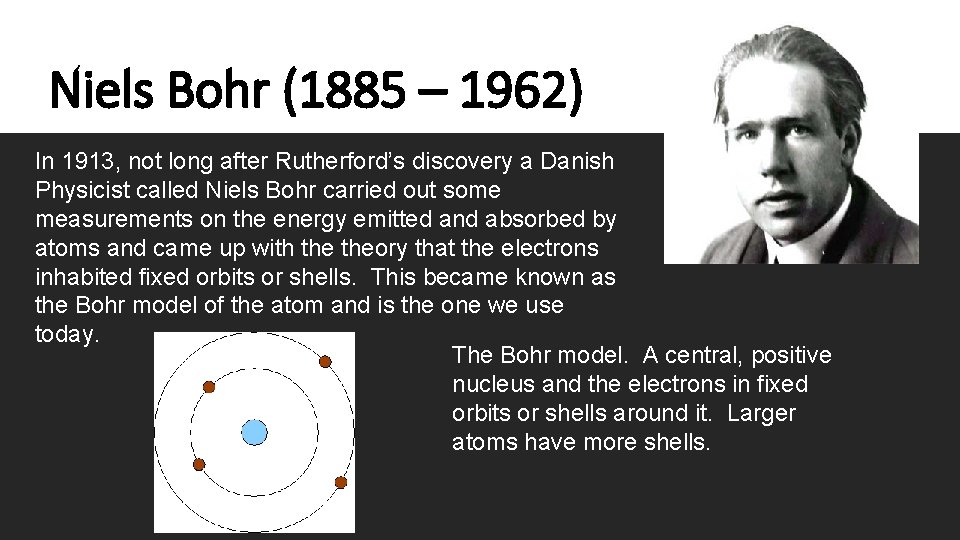
Niels Bohr (1885 – 1962) In 1913, not long after Rutherford’s discovery a Danish Physicist called Niels Bohr carried out some measurements on the energy emitted and absorbed by atoms and came up with theory that the electrons inhabited fixed orbits or shells. This became known as the Bohr model of the atom and is the one we use today. The Bohr model. A central, positive nucleus and the electrons in fixed orbits or shells around it. Larger atoms have more shells.

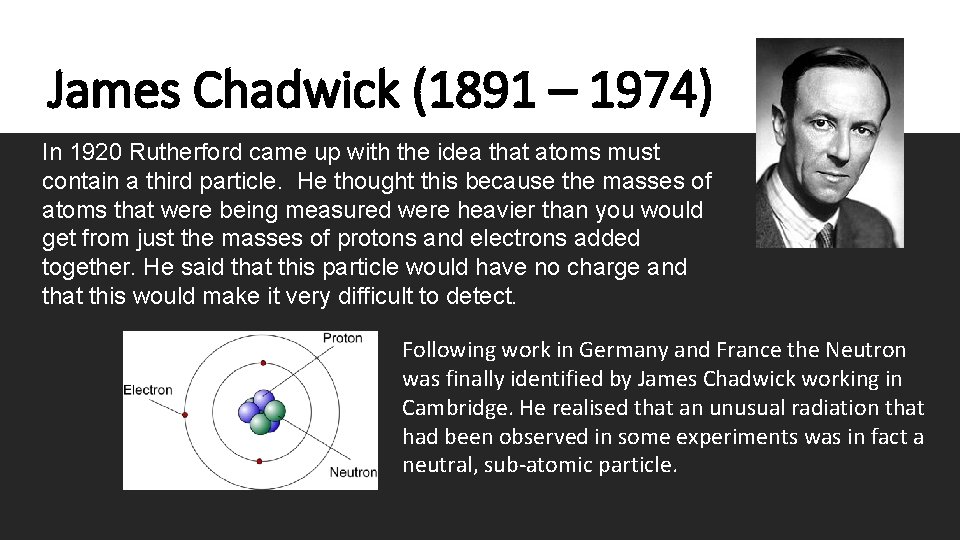
James Chadwick (1891 – 1974) In 1920 Rutherford came up with the idea that atoms must contain a third particle. He thought this because the masses of atoms that were being measured were heavier than you would get from just the masses of protons and electrons added together. He said that this particle would have no charge and that this would make it very difficult to detect. Following work in Germany and France the Neutron was finally identified by James Chadwick working in Cambridge. He realised that an unusual radiation that had been observed in some experiments was in fact a neutral, sub-atomic particle.

Back to Rutherford’s Alpha Scattering Experiment The is one of the most important experiments in the history of science. Professor Rutherford wanted to see what happened to alpha particles when they collided with atoms. He asked two of his students (Hans Geiger and Ernest Marsden) to carry out the experiment.

The Alpha Scattering Experiment You are going to work in groups of 3 or 4 to figure out the details of the experiments and its results: • Some information about the experiment is stuck up outside the room. • You will have 4 opportunities to collect information. • Taking it in turn, one person, a runner, will leave the room and look at the information. • The first time, the runner will have 40 s. • The second time 30 s, third time 20 s, and final time 10 s. • You will have 2 min in between each run to compile your information.

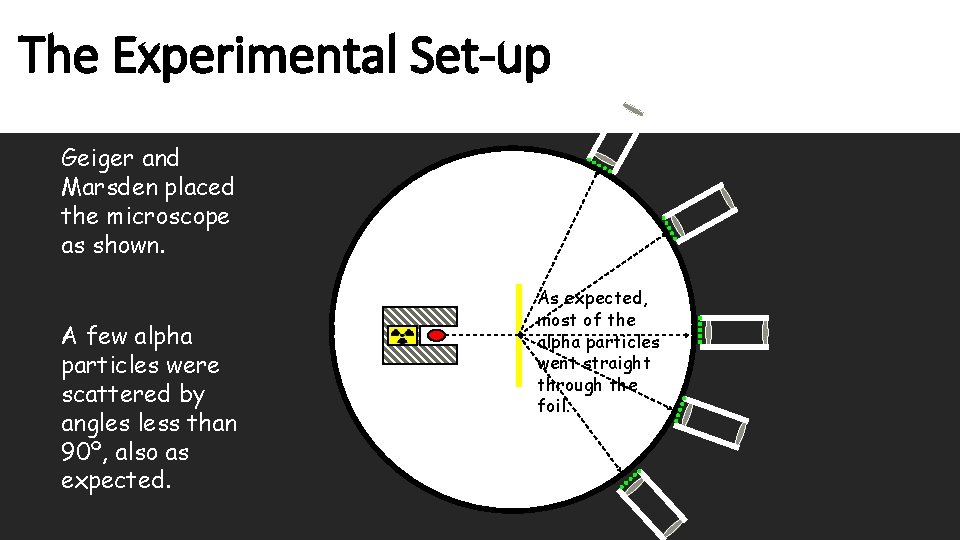
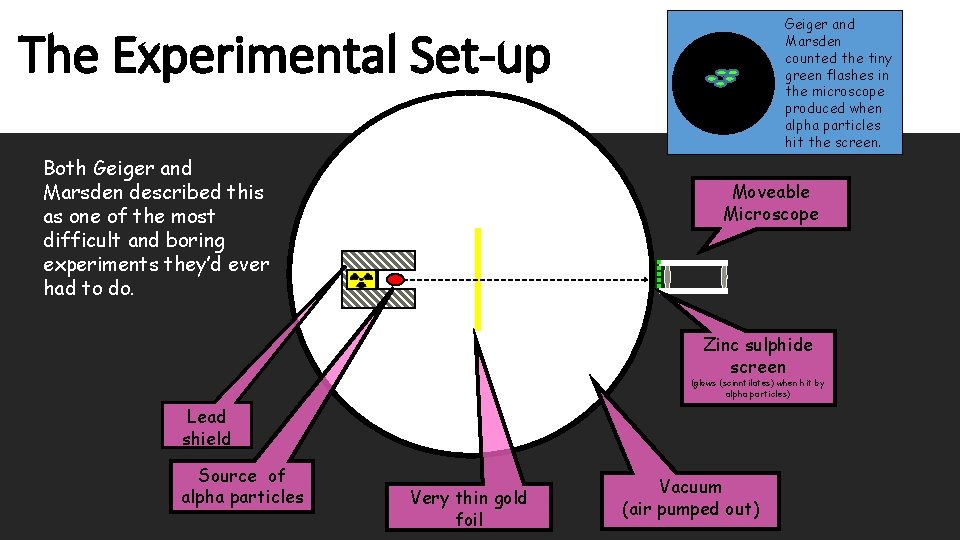
The Experimental Set-up Geiger and Marsden placed the microscope as shown. A few alpha particles were scattered by angles less than 90º, also as expected. As expected, most of the alpha particles went straight through the foil.

Geiger and Marsden counted the tiny green flashes in the microscope produced when alpha particles hit the screen. The Experimental Set-up Both Geiger and Marsden described this as one of the most difficult and boring experiments they’d ever had to do. Moveable Microscope Zinc sulphide screen (glows (scinntilates) when hit by alpha particles) Lead shield Source of alpha particles Very thin gold foil Vacuum (air pumped out)

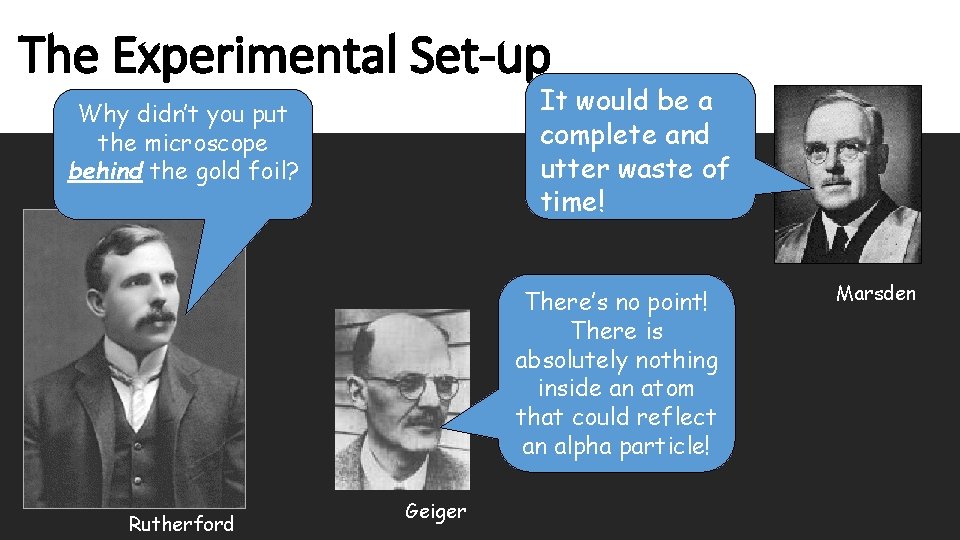
The Experimental Set-up It would be a complete and utter waste of time! Why didn’t you put the microscope behind the gold foil? There’s no point! There is absolutely nothing inside an atom that could reflect an alpha particle! Rutherford Geiger Marsden

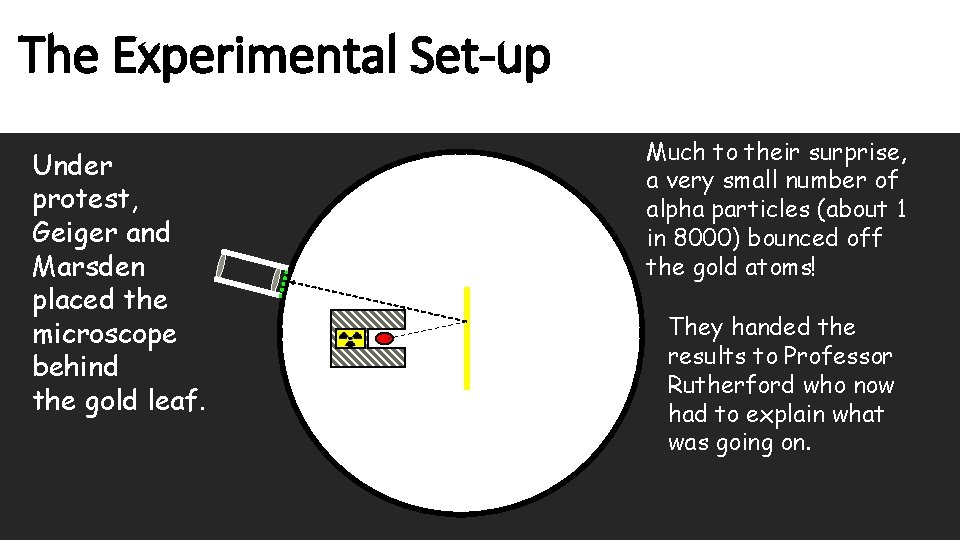
The Experimental Set-up Under protest, Geiger and Marsden placed the microscope behind the gold leaf. Much to their surprise, a very small number of alpha particles (about 1 in 8000) bounced off the gold atoms! They handed the results to Professor Rutherford who now had to explain what was going on.

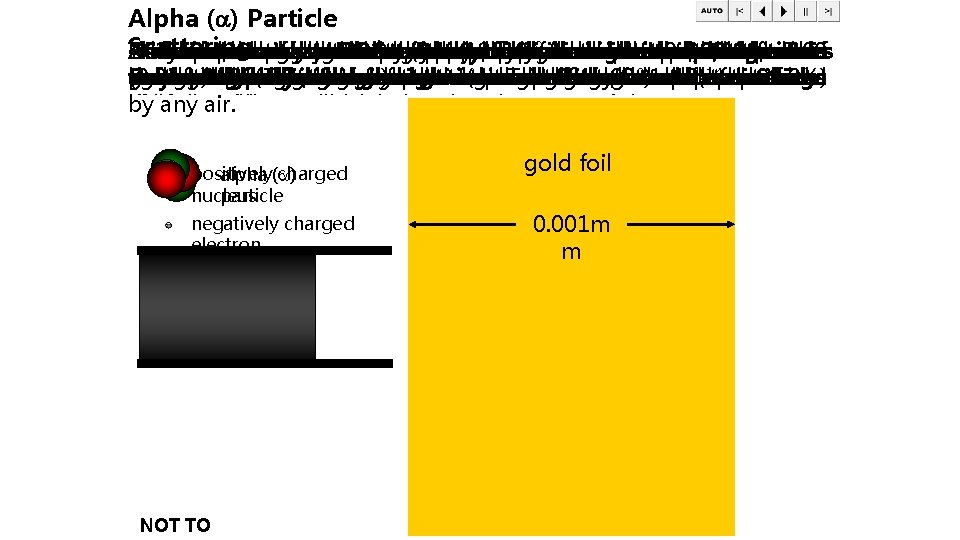
Alpha ( ) Particle th -9 students: Scattering Athas An A They However, He Due Rutherford It The If Air an fluorescent the also was experiment actual diameter to atom observed fired since start the pumped knew awork alpha were knew suggested been small tiny of of that screen by the was athe that discovered particles out minority, gold number the Ernest the 20 done size of alpha that negatively atom vast was the of century, by (from Rutherford of most about particles this majority that apparatus placed two isalpha 0. 3 x 10 screen, athe of of charged physicists one radioactive his the particles around had nucleus of m to discovered in the masses which create electrons eight nucleus alpha the knew isthat source) of over is. Hans equal a thousand, foil, the particles 0. 3 were alpha that vacuum. would 10, 000 had atom nanometres that Geiger at tothe deflected too sheet those particle be passed is would times atom were little This in and 100 of a 0 the -14 particle. contained scattering. Ernest gold produce straight deflected light mass through tiny smaller (0. 3 nm). times was to foil atoms to positively smaller Marsden, done than through deflect (gold a these The through flash positive (e. g. the This to diameter than leaf) large the ensure of size helium) the charged who gave light large aalpha that and full foil of angles, were of that a an whenever stop. angles with was negative so clearer the particles. atom centre later the whatever one this anucleus few (greater alpha and to thousandth insight meant itcharges undergoing called make was Therefore, most is deflected particles less hit than that into significant of the by but than an of there 90 the an the were small the nucleus a atom ). 1 x 10 alpha millimetre structure deflection must alpha structure not contributions deflections. (over m. absorbed be and particles 99. 9%) of thick. large must was the still atom. in At must be distances electrons is by empty nuclear this any due not have width air. to understood. space. form the physics. between more itpositive a is mass. cloud about these charges. within 3, 000 positive the atoms ‘space’ charges. across. of the atom. gold foil fluorescent positively charged alpha ( ) nucleus particle negatively charged electron screen 0. 001 m gold foil (0. 001 mmm thick) radioactive source NOT TO

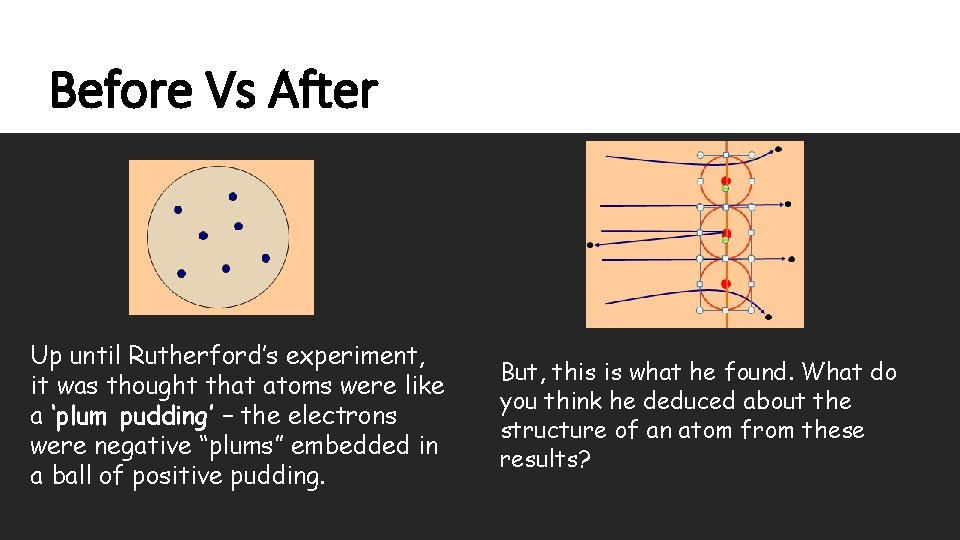
Before Vs After Up until Rutherford’s experiment, it was thought that atoms were like a ‘plum pudding’ – the electrons were negative “plums” embedded in a ball of positive pudding. But, this is what he found. What do you think he deduced about the structure of an atom from these results?

Nobel Prize The top scientific honour in the world is the Nobel Prize. Ernest Rutherford was awarded the Nobel Prize in 1908 for discovering the atomic nucleus. (He deserved it – it was his calculations based on the data that measured the size of the nucleus. Geiger and Marsden were given full credit in the published scientific paper. ) However, he was slightly disappointed because he was given the Nobel Prize for Chemistry instead of Physics All science is either Physics or stamp collecting. Rutherford