Atomic Structure and Periodicity Electromagnetic Radiation The Nature





























- Slides: 43

Atomic Structure and Periodicity Electromagnetic Radiation The Nature of Matter The Atomic Spectrum of Hydrogen The Bohr Model The Quantum Mechanical Model


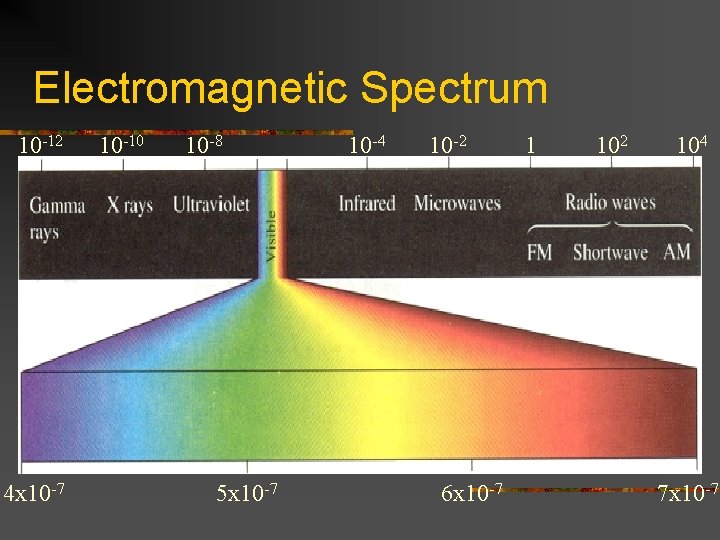
Electromagnetic Radiation n n n n Gamma X rays Ultraviolet Visible (400 -700 nm) Infrared Microwaves Radio waves Power waves

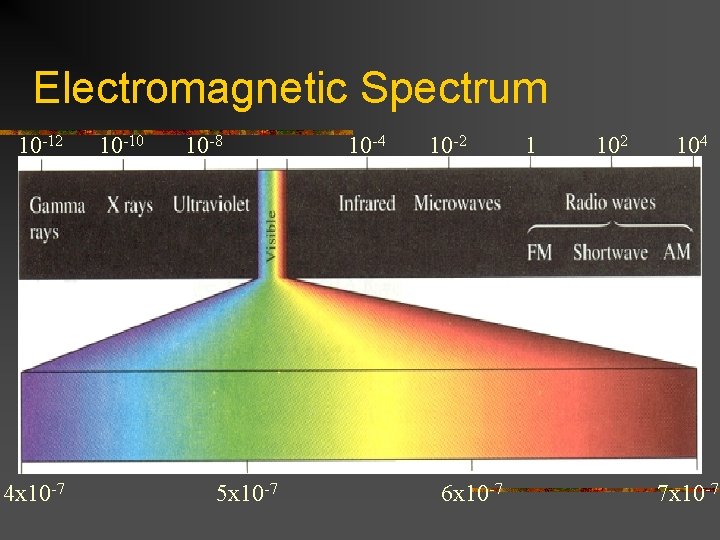
Electromagnetic Spectrum 10 -12 4 x 10 -7 10 -10 10 -8 5 x 10 -7 10 -4 10 -2 6 x 10 -7 1 102 104 7 x 10 -7

Electromagnetic Radiation n Three primary characteristics n n n Wavelength ( …lambda) Frequency ( …nu) Speed (c)

Wavelength n n Distance between two consecutive crests or troughs of a wave Measured in m or nm, typically

Frequency n n n Number of wave cycles per second that pass a given point in space Cycle is understood in SI language Measured in 1/s or s-1, also known as a hertz (Hz)

Speed n n Constant, known as the speed of light 2. 9979 x 108 m/s Since the speed of a wave is constant, then frequency and wavelength must vary inversely c =

Problem #1 n A wave is known to have a frequency of 5. 09 x 1014 Hz. What is its wavelength and what type of electromagnetic radiation is it?

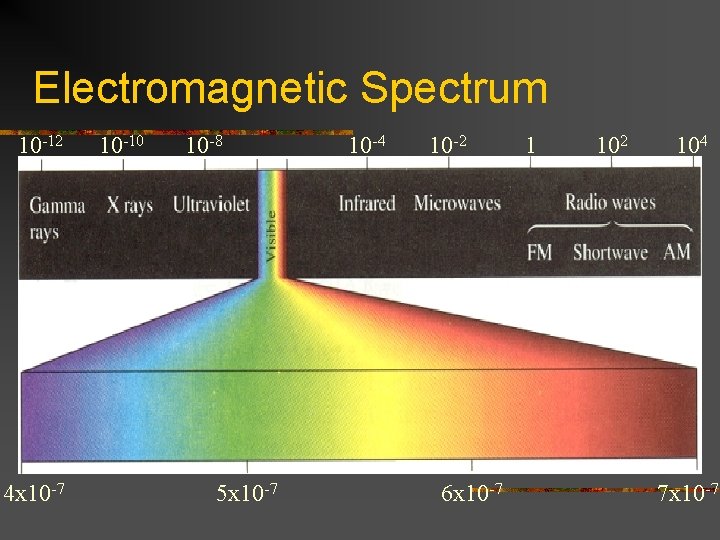
Electromagnetic Spectrum 10 -12 4 x 10 -7 10 -10 10 -8 5 x 10 -7 10 -4 10 -2 6 x 10 -7 1 102 104 7 x 10 -7

Problem #1 n A wave is known to have a frequency of 5. 09 x 1014 Hz. What is its wavelength and what type of electromagnetic radiation is it? 5. 89 x 10 -7 m Visible yellow

The Nature of Matter n Matter and energy (in the form of light) were thought to be distinct until 1900 n n Matter was made of particles that had mass, took up space, and could absorb or emit any quantity of energy Light was made of waves that were massless and of unknown location (delocalized)

Max Planck (1858 -1947) n n German physicist Observed that heated solid bodies emitted energy only in specific whole-number multiples They were multiples of the quantity “h ” h is known as Planck’s constant and has a value of 6. 626 x 10 -34 J • s

Max Planck (1858 -1947) n Thus, the change in internal energy of a system is represented by n n n E = h “h ” came to be known as a quantum Proved that energy is indeed quantized not continuous

Problem #2 n Cuprous ions will emit 4. 41 x 10 -19 J when heated to approximately 1200 C. What is the wavelength of the light emitted and what color is it?

Electromagnetic Spectrum 10 -12 4 x 10 -7 10 -10 10 -8 5 x 10 -7 10 -4 10 -2 6 x 10 -7 1 102 104 7 x 10 -7

Problem #2 n Cuprous ions will emit 4. 41 x 10 -19 J when heated to approximately 1200 C. What is the wavelength of the light emitted and what color is it? 4. 50 x 10 -7 m blue

Albert Einstein n Proposed the electromagnetic radiation may be viewed as a stream of particles, known as “photons” Said that the energy of a photon equaled the change in internal energy that a system experienced Ephoton= h = hc/

Albert Einstein n n In 1905, he proposed that energy has mass and put forth the famed equation E = mc 2 or m = E/c 2 Thus, m = E = hc/ = h c 2 c Established the phrase “dual nature of light”

Prince Louis-Victor Pierre Raymond de Broglie n n n Proved that the opposite of the dual nature of light was true Showed that particles also exhibited wave properties de Broglie’s equation replaces the speed of light with the speed of the particle m= h or = h v mv

Problem #3 n Compare the wavelength of an electron with a mass of 9. 11 x 10 -31 kg traveling at a speed of 1. 00 x 107 m/s with that of a tennis ball with a mass of 0. 0089 kg traveling at 42. 5 m/s. Electron— 7. 27 x 10 -11 m Tennis ball— 1. 75 x 10 -33 m

Diffraction n Scattering of light from a regular array of points or lines. . make a diffraction pattern Proves the wave properties of particulate matter Pattern results from constructive interference n n Light spots And destructive interference n Dark spots

Matter n n Exhibits particulate and wave properties Big bits have tiny wavelengths and have more particulate properties Itty-bitty bits have larger wavelengths and behave more like waves than particles Medium bits have fairly equal representation of particles and waves

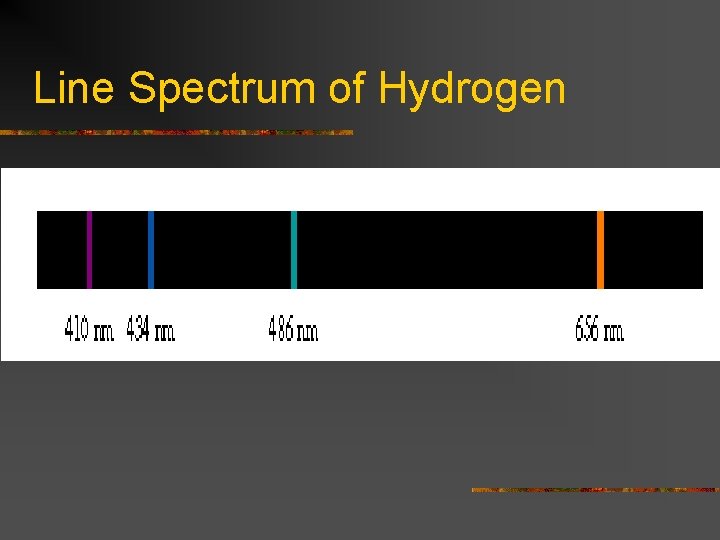
Atomic Spectrum of Hydrogen n n When H atoms are excited, they emit the excess energy according to the electromagnetic spectrum This is known as an emission spectrum It is not continuous as white light through a prism is Rather, it is known as a line spectrum Verifies quantization of energy emission

Line Spectrum of Hydrogen

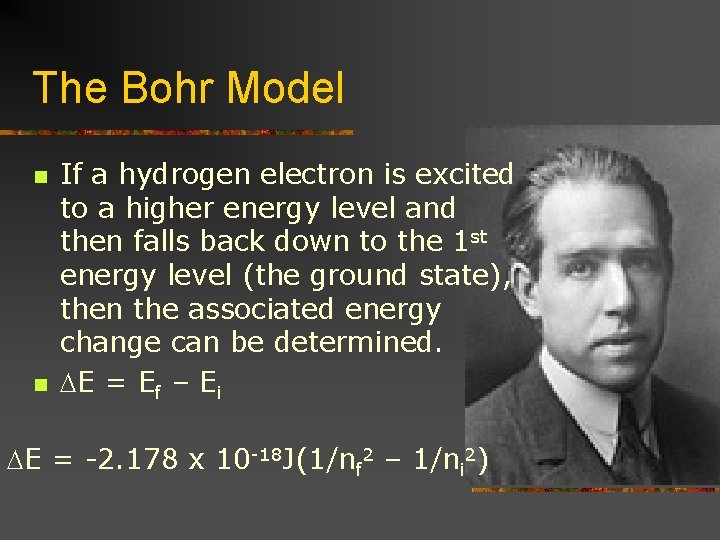
The Bohr Model n n n developed in 1913 by Danish physicist, Niels Bohr Proposed that the electron in H moves in particular circular orbits Agreed with the emission spectrum of hydrogen assuming the angular momentum of the electron occurred in specific increments

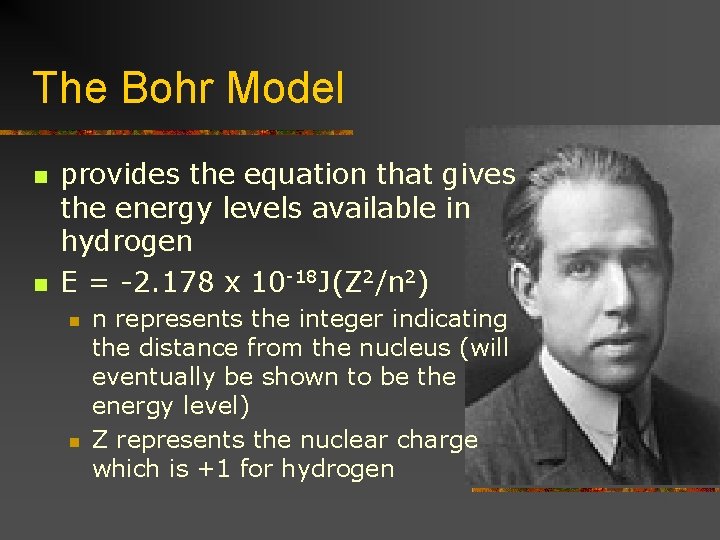
The Bohr Model n n provides the equation that gives the energy levels available in hydrogen E = -2. 178 x 10 -18 J(Z 2/n 2) n n n represents the integer indicating the distance from the nucleus (will eventually be shown to be the energy level) Z represents the nuclear charge which is +1 for hydrogen

The Bohr Model n n If a hydrogen electron is excited to a higher energy level and then falls back down to the 1 st energy level (the ground state), then the associated energy change can be determined. E = Ef – Ei E = -2. 178 x 10 -18 J(1/nf 2 – 1/ni 2)

Problem #4 n Determine the wavelength of light emitted when a hydrogen electron falls from the 6 th energy level to the 1 st energy level. What type of electromagnetic radiation is this? 9. 38 x 10 -8 m ultraviolet

The Quantum Mechanical Model n n Begun by de Broglie Remember the dual nature of light and the idea that all matter traveled in waves and as particles?

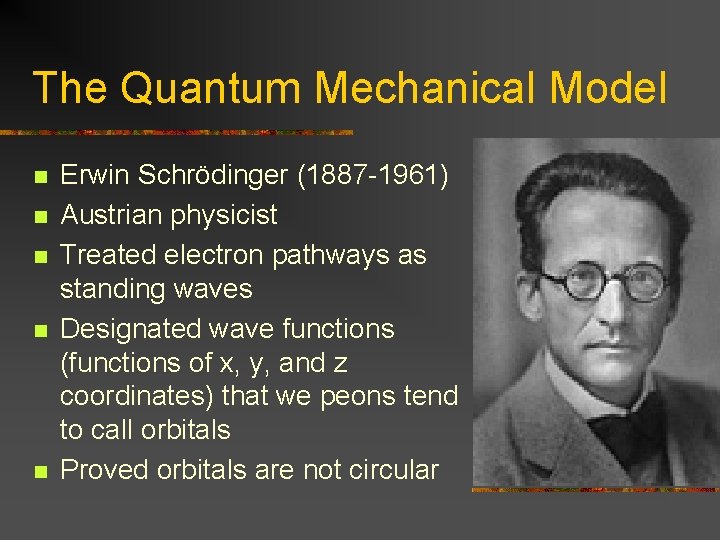
The Quantum Mechanical Model n n n Erwin Schrödinger (1887 -1961) Austrian physicist Treated electron pathways as standing waves Designated wave functions (functions of x, y, and z coordinates) that we peons tend to call orbitals Proved orbitals are not circular

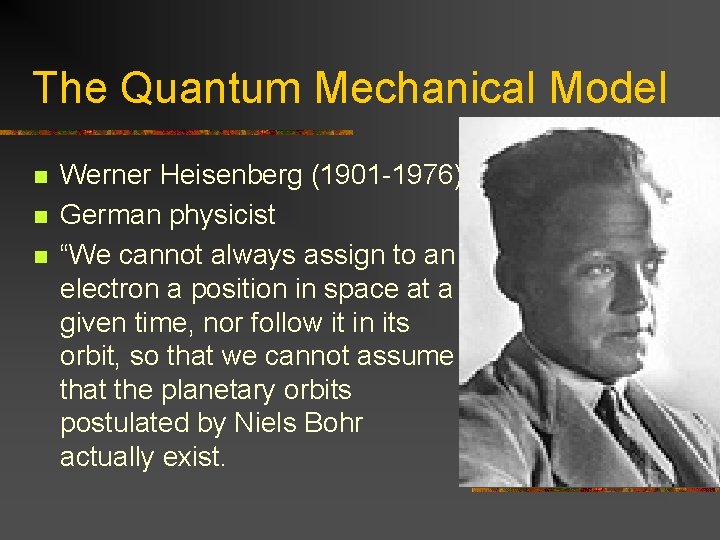
The Quantum Mechanical Model n n n Werner Heisenberg (1901 -1976) German physicist “We cannot always assign to an electron a position in space at a given time, nor follow it in its orbit, so that we cannot assume that the planetary orbits postulated by Niels Bohr actually exist.

The Quantum Mechanical Model n n n Mechanical quantities, such as position, velocity, etc. should be represented, not by ordinary numbers, but by abstract mathematical structures called matrices. “ Proposed the above postulate at the age of 23!! Later came up with his famed Uncertainty Theory

Heisenberg’s Uncertainty Principle n n There is a fundamental limitation to just how precisely we can know both the position and momentum of a particle at a given time. x • (mv) > h/4 n n n x is the uncertainty in position (mv) is the uncertainty in momentum h is Planck’s constant

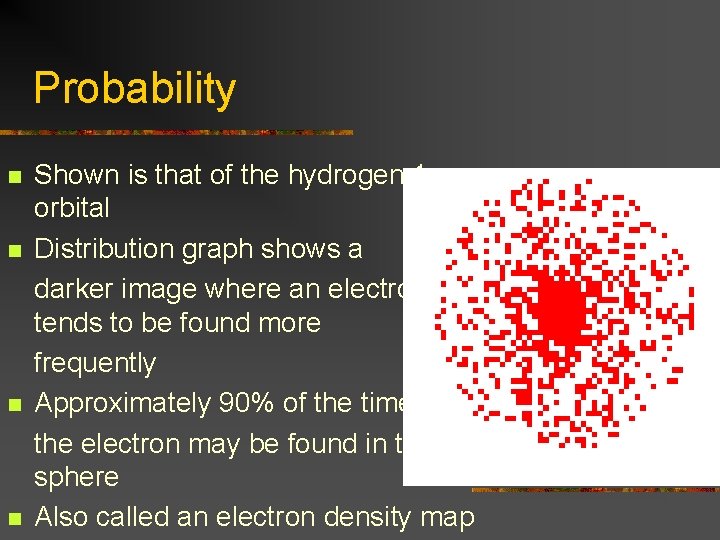
Probability n n Shown is that of the hydrogen 1 s orbital Distribution graph shows a darker image where an electron tends to be found more frequently Approximately 90% of the time, the electron may be found in this sphere Also called an electron density map

Quantum Numbers n n n Give the address of an electron by describing the orbital within which it spends some time Four numbers assigned for every electron of every atom Principal Angular momentum or azimuthal Magnetic Spin

Principal Quantum Number n n Represented by “n” May be any positive integer (1, 2, 3…) Describes the size of the orbital and its energy The larger the number, the farther away from the nucleus and the less tightly bound the electron is to the nucleus

Angular Momentum (or Azimuthal) Quantum Number n n n Represented by “l” May be any positive integer from 0 to (n-1) Describes the shape of the orbital or the pathway that the electron takes Dependent upon the principal quantum number Corresponds to sublevels within energy levels

Magnetic Quantum Number n Represented by “ml” n May be any integer from – l to +l n n n Describes the orientation in three dimensional space of the orbital Dependent upon the principal quantum number and the azimuthal quantum number Corresponds to the coordinate at which we find particular orbitals

Spin Quantum Number n n Represented by “ms” May be –½ or +½ Describes the spin of the electron w I thin its orbital Dependent upon the other electron’s spin number

Quantum Numbers n What are the four quantum numbers of hydrogen’s electron? n=1 l=0 ml = 0 ms = –½

Quantum Numbers n What are the four quantum numbers of all of boron’s five electrons? n=1 l=0 ml = 0 ms = –½ n=1 l=0 ml = 0 ms = +½ n=2 l=0 ml = 0 ms = -½ n=2 l=0 ml = 0 ms = +½ n=2 l=1 ml = -1 ms = -½

Quantum Numbers n What are the four quantum numbers of sodium’s valence electron? n=3 l=0 ml = 0 ms = –½