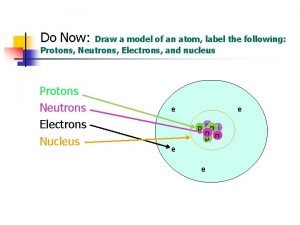
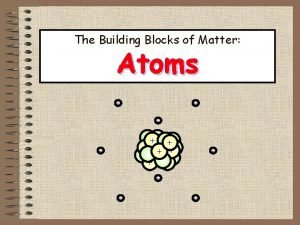
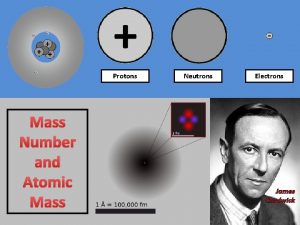
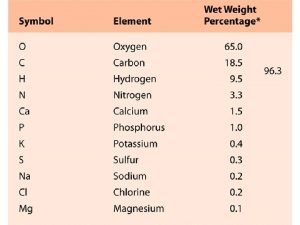
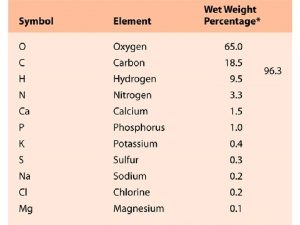
Atomic Structure and Periodicity Atoms Protons Neutrons Electrons















































- Slides: 73

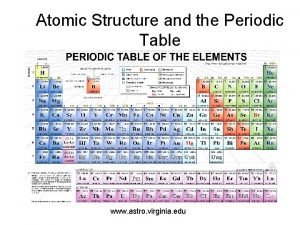
Atomic Structure and Periodicity

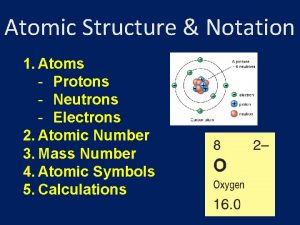
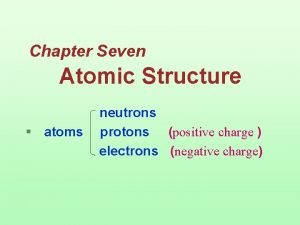
Atoms Protons Neutrons Electrons 1. Where are the electrons 2. Do they have different energies



Electromagnetic Radiation Radiant energy that exhibits wavelength-like behavior and travels through space at the speed of light in a vacuum.


Waves have 3 primary characteristics: 1. Wavelength: distance between two peaks in a wave. 2. Frequency: number of waves per second that pass a given point in space. 3. Speed: speed of light is 2. 9979 108 m/s.


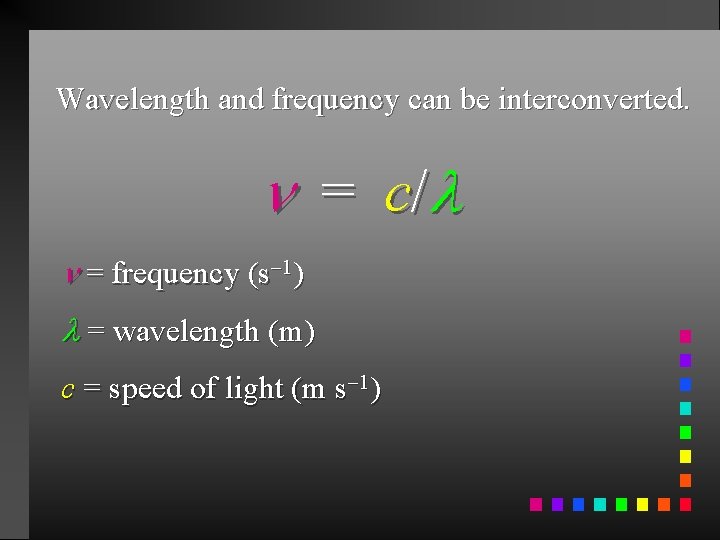
Wavelength and frequency can be interconverted. = c/ = frequency (s 1) = wavelength (m) c = speed of light (m s 1)


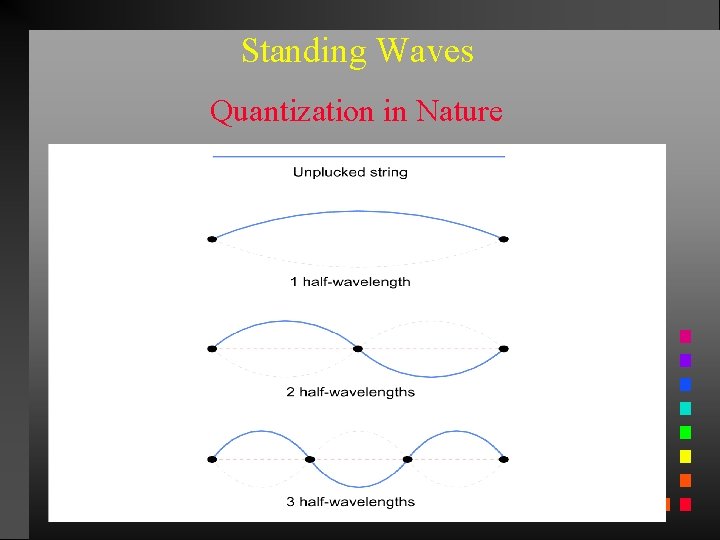
Standing Waves Quantization in Nature


Planck’s Constant Transfer of energy is quantized, and can only occur in discrete units, called quanta. E = change in energy, in J h = Planck’s constant, 6. 626 10 34 J s = frequency, in s 1 = wavelength, in m

Energy and Mass Energy has mass E = mc 2 E = energy m = mass c = speed of light

Energy and Mass (Hence the dual nature of light. )

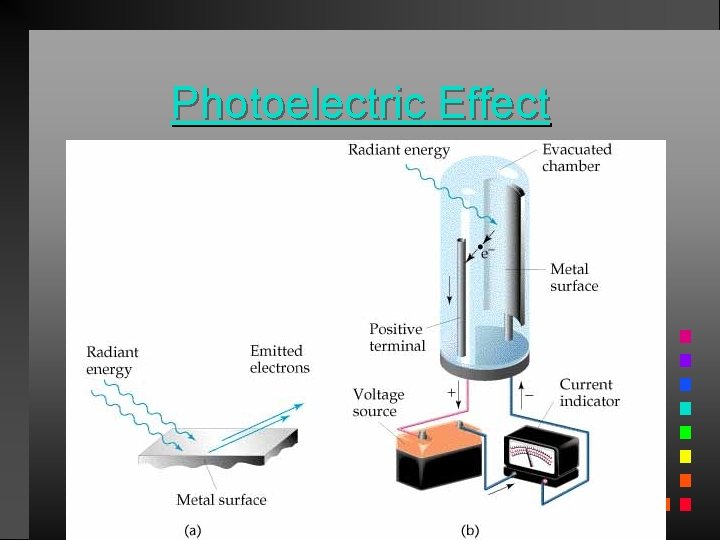
Photoelectric Effect

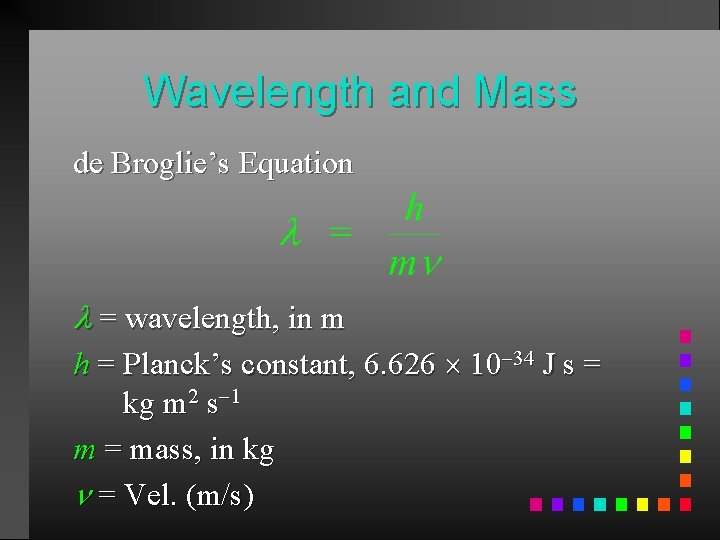
Wavelength and Mass de Broglie’s Equation = wavelength, in m h = Planck’s constant, 6. 626 10 34 J s = kg m 2 s 1 m = mass, in kg = Vel. (m/s)

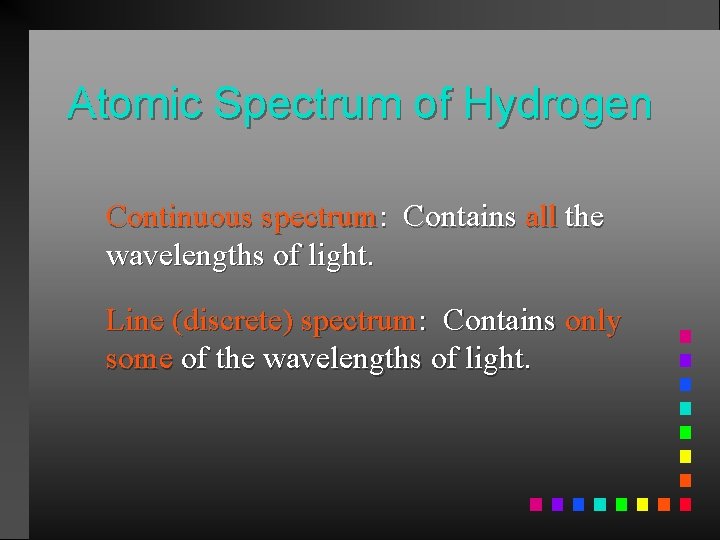
Atomic Spectrum of Hydrogen Continuous spectrum: Contains all the wavelengths of light. Line (discrete) spectrum: Contains only some of the wavelengths of light.



The Bohr Model The electron in a hydrogen atom moves around the nucleus only in certain allowed circular orbits. E = energy of the levels in the H-atom z = nuclear charge (for H, z = 1) n = an integer

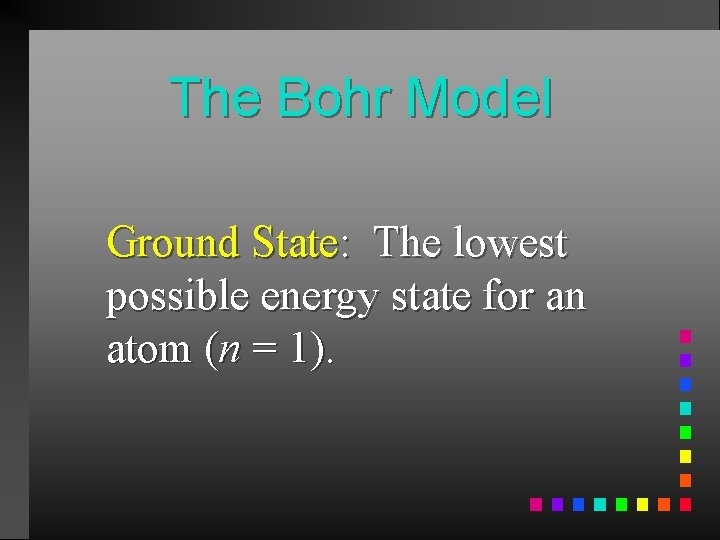
The Bohr Model Ground State: The lowest possible energy state for an atom (n = 1).

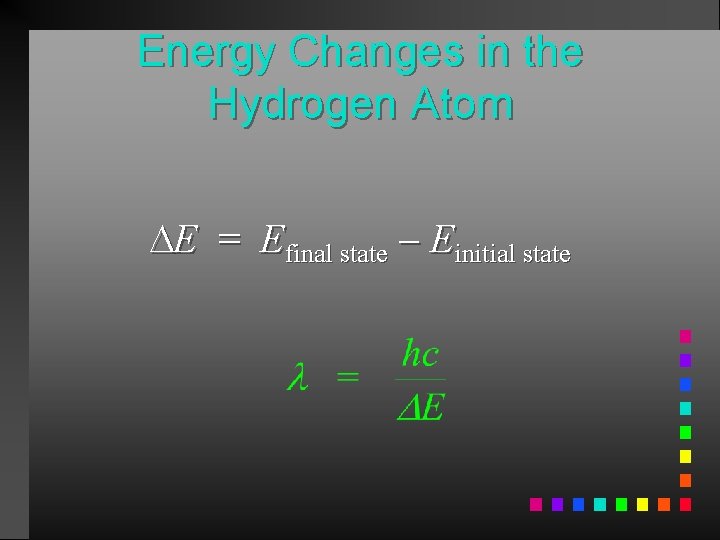
Energy Changes in the Hydrogen Atom E = Efinal state Einitial state



Atomic Spectrum

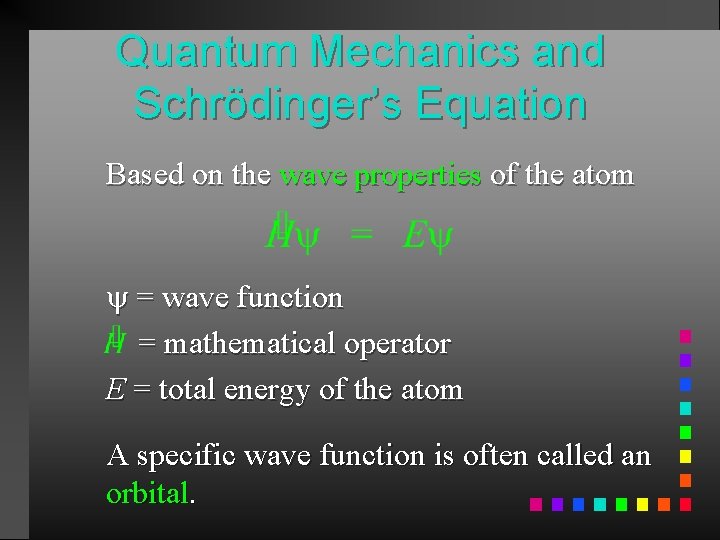
Quantum Mechanics and Schrödinger’s Equation Based on the wave properties of the atom = wave function = mathematical operator E = total energy of the atom A specific wave function is often called an orbital.

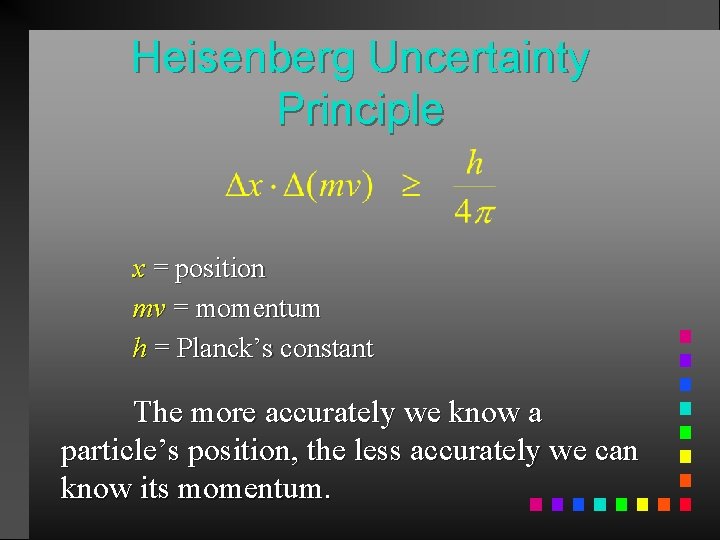
Heisenberg Uncertainty Principle x = position mv = momentum h = Planck’s constant The more accurately we know a particle’s position, the less accurately we can know its momentum.

Probability Distribution - square of the wave function (Ψ 2) - probability of finding an electron at a given position Radial probability distribution is the probability distribution in each spherical shell.

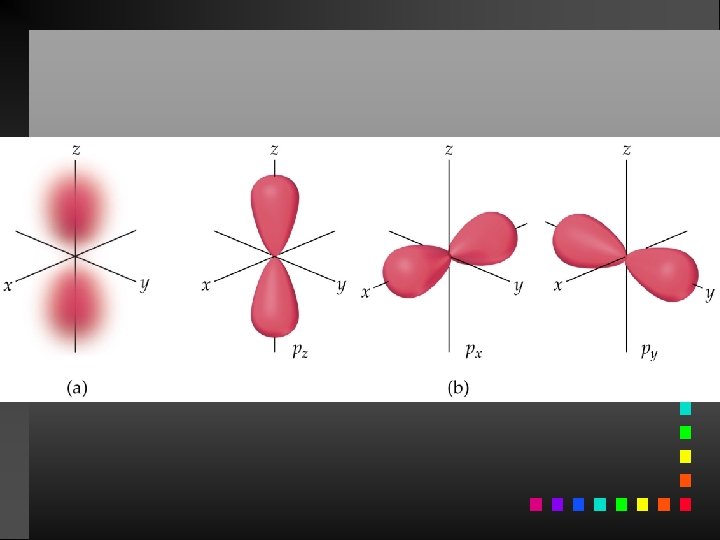
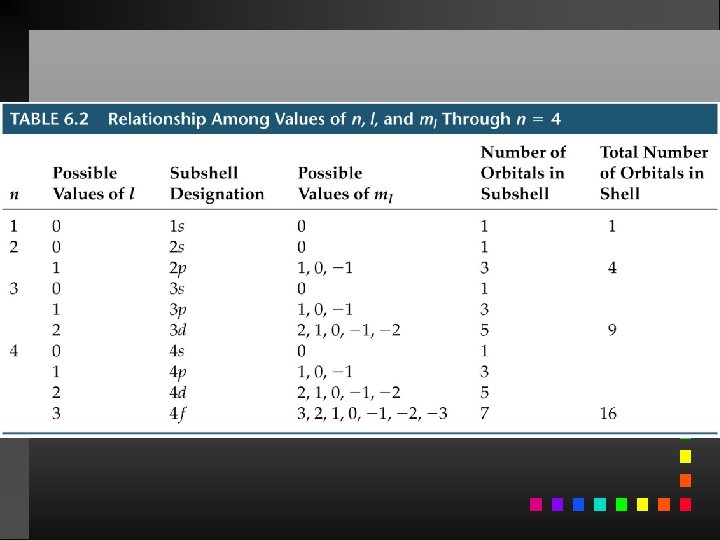
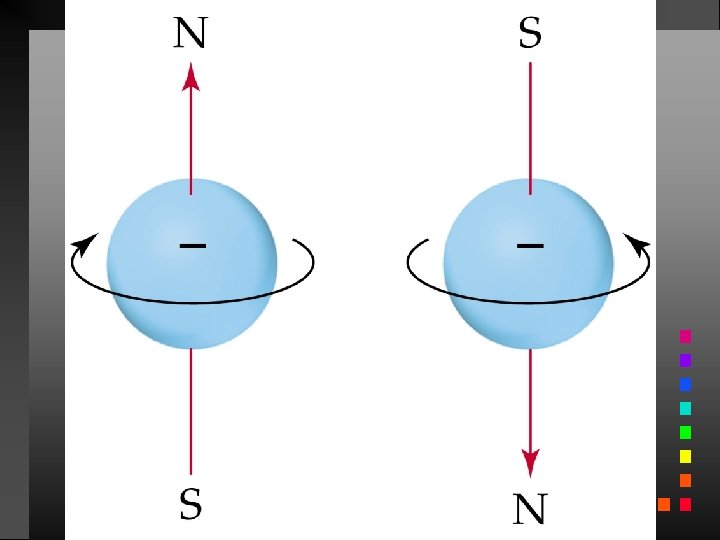
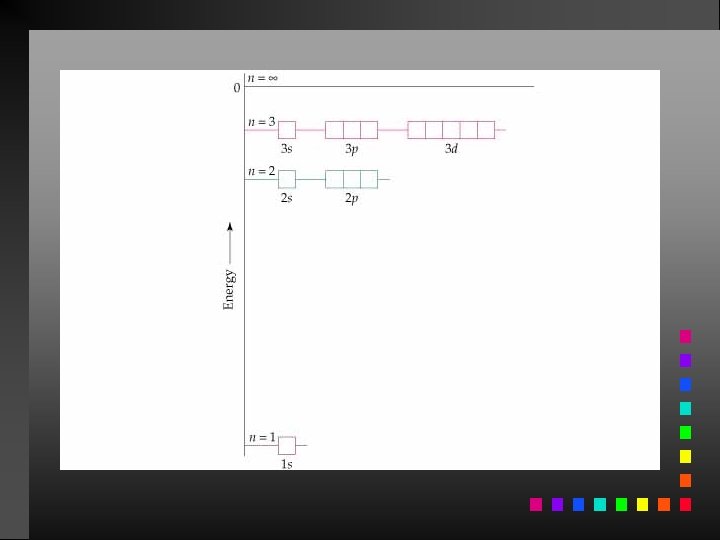
Quantum Numbers (QN) 1. Principal QN (n = 1, 2, 3, . . . ) - related to size and energy of the orbital. 2. Angular Momentum QN (l = 0 to n 1) - relates to shape of the orbital. 3. Magnetic QN (ml = l to l) - relates to orientation of the orbital in space relative to other orbitals. 4. Electron Spin QN (ms = +1/2, 1/2) - relates to the spin states of the electrons.





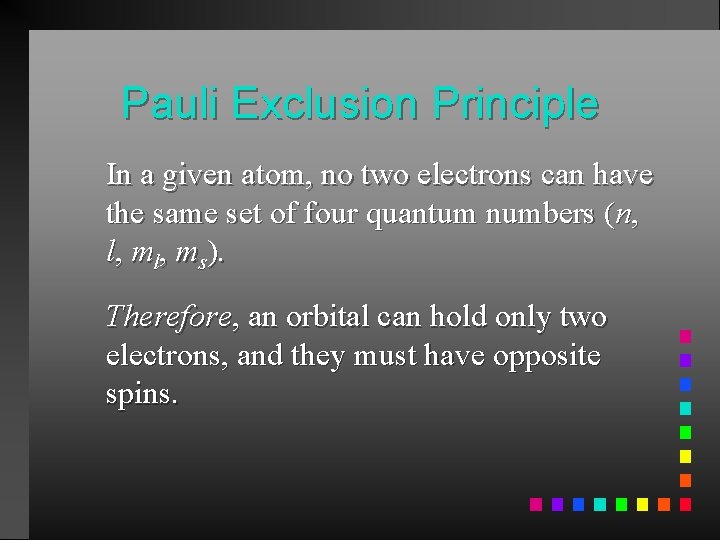
Pauli Exclusion Principle In a given atom, no two electrons can have the same set of four quantum numbers (n, l, ms). Therefore, an orbital can hold only two electrons, and they must have opposite spins.


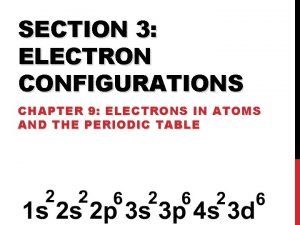
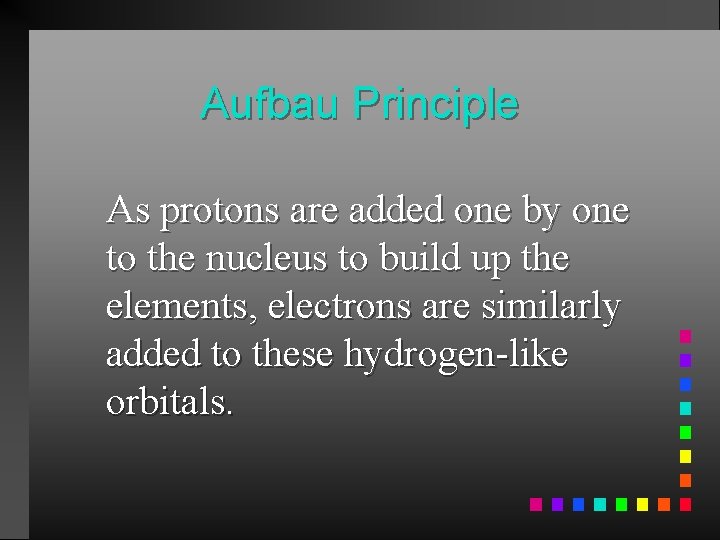
Aufbau Principle As protons are added one by one to the nucleus to build up the elements, electrons are similarly added to these hydrogen-like orbitals.



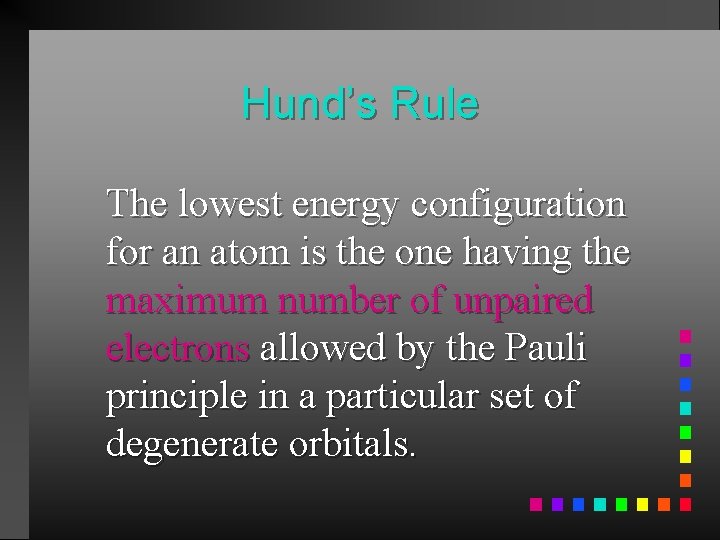
Hund’s Rule The lowest energy configuration for an atom is the one having the maximum number of unpaired electrons allowed by the Pauli principle in a particular set of degenerate orbitals.


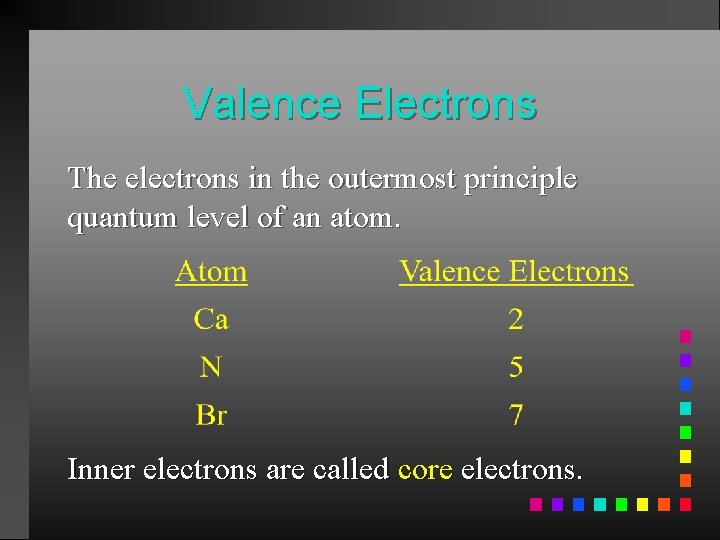
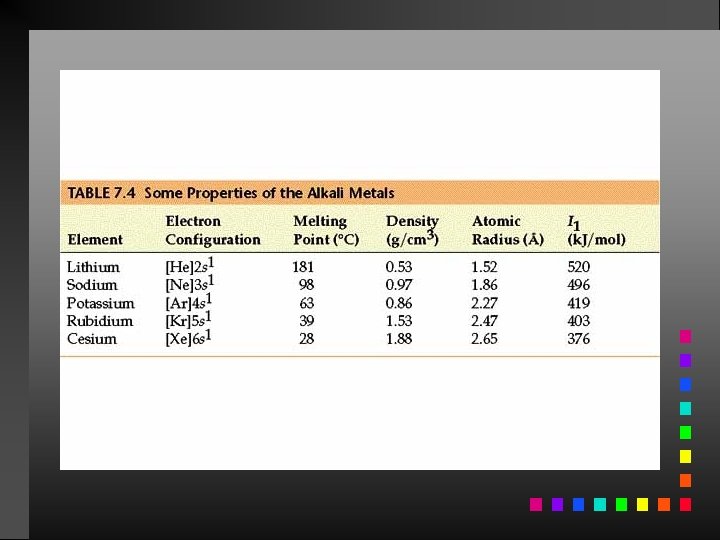
Valence Electrons The electrons in the outermost principle quantum level of an atom. Inner electrons are called core electrons.



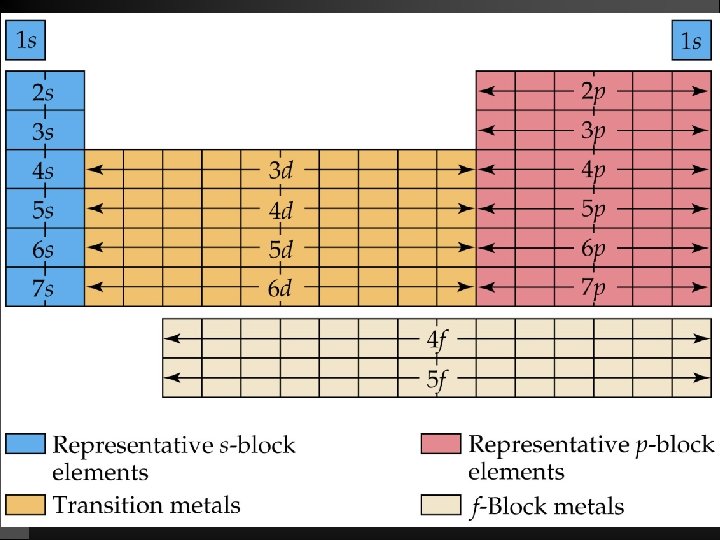
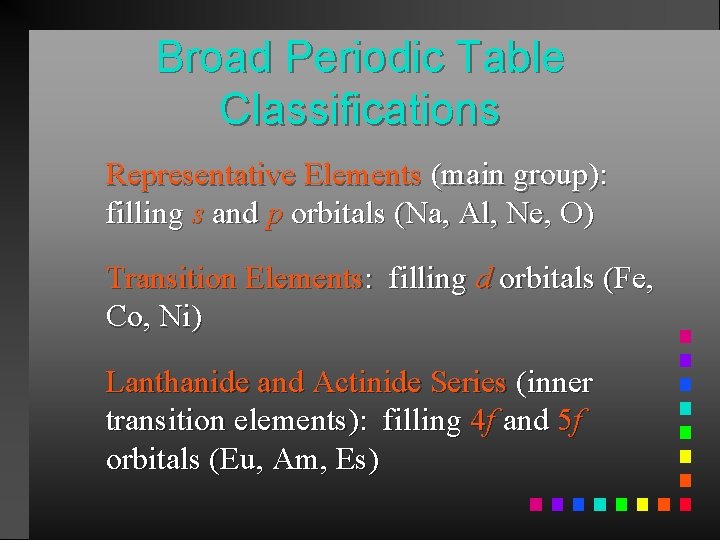
Broad Periodic Table Classifications Representative Elements (main group): filling s and p orbitals (Na, Al, Ne, O) Transition Elements: filling d orbitals (Fe, Co, Ni) Lanthanide and Actinide Series (inner transition elements): filling 4 f and 5 f orbitals (Eu, Am, Es)

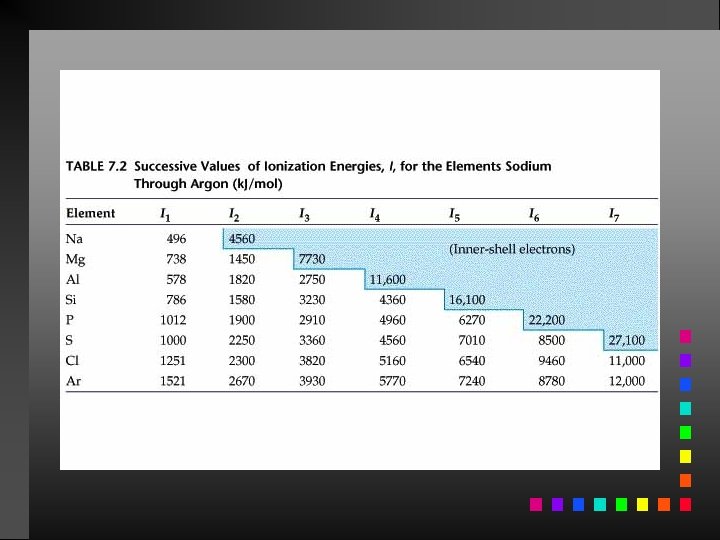
Ionization Energy The quantity of energy required to remove an electron from the gaseous atom or ion.

Periodic Trends First ionization energy: increases from left to right across a period; decreases going down a group.


Periodic Trends Atomic Radii: decrease going from left to right across a period; increase going down a group.


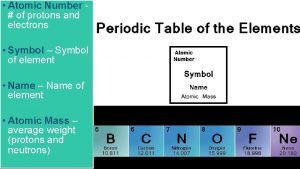
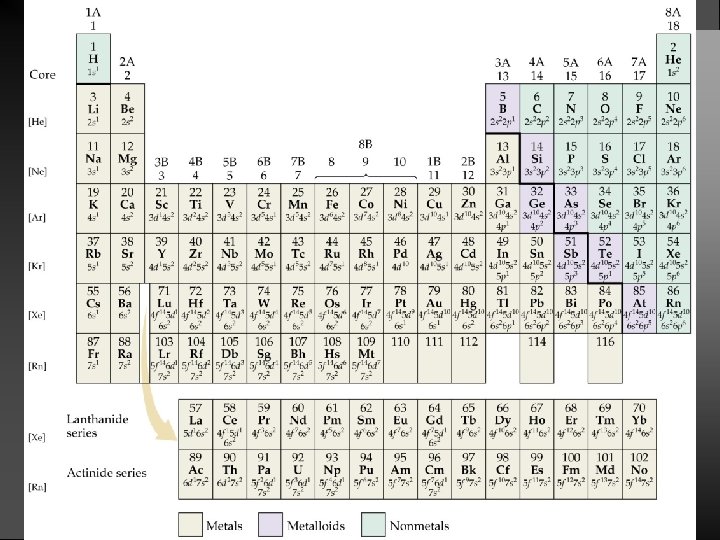
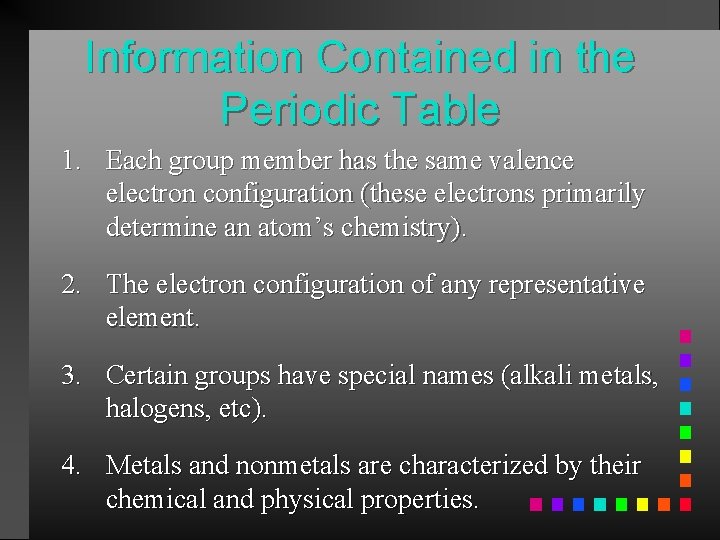
Information Contained in the Periodic Table 1. Each group member has the same valence electron configuration (these electrons primarily determine an atom’s chemistry). 2. The electron configuration of any representative element. 3. Certain groups have special names (alkali metals, halogens, etc). 4. Metals and nonmetals are characterized by their chemical and physical properties.

END




















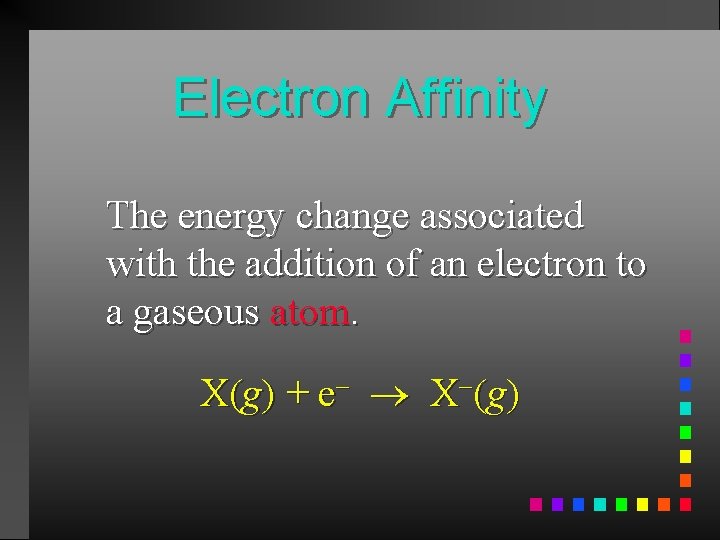
Electron Affinity The energy change associated with the addition of an electron to a gaseous atom. X(g) + e X (g)

photo
 Sulfur number of neutrons protons and electrons
Sulfur number of neutrons protons and electrons How to find number of protons
How to find number of protons Ytterbium atomic number
Ytterbium atomic number Arrangement of subatomic particles
Arrangement of subatomic particles What is an atom inventory
What is an atom inventory Introduction to basic chemistry
Introduction to basic chemistry Lithium protons neutrons electrons
Lithium protons neutrons electrons Chromium 58 neutrons
Chromium 58 neutrons 12c6 number of protons and neutrons
12c6 number of protons and neutrons Timeline of democritus
Timeline of democritus 39k+ protons neutrons electrons
39k+ protons neutrons electrons 39k+ protons neutrons electrons
39k+ protons neutrons electrons Describe neutrons.location: charge: mass:
Describe neutrons.location: charge: mass: Chlorine protons neutrons electrons
Chlorine protons neutrons electrons Ap chemistry atomic structure and periodicity
Ap chemistry atomic structure and periodicity Ap chemistry chapter 7 atomic structure and periodicity
Ap chemistry chapter 7 atomic structure and periodicity Chapter 7 atomic structure and periodicity
Chapter 7 atomic structure and periodicity Protons and neutrons size
Protons and neutrons size Lithium number of protons
Lithium number of protons Element scavenger hunt
Element scavenger hunt Can an atom have more neutrons than protons
Can an atom have more neutrons than protons Can an atom have more neutrons than protons
Can an atom have more neutrons than protons Can an atom have more neutrons than protons
Can an atom have more neutrons than protons Os atomos sao formados por protons neutrons e eletrons
Os atomos sao formados por protons neutrons e eletrons Element name
Element name Non metal halogen
Non metal halogen More protons than electrons
More protons than electrons Is the atomic number the number of protons
Is the atomic number the number of protons Two protons in an atomic nucleus are typically
Two protons in an atomic nucleus are typically Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Lowest allowable energy state of an atom
Lowest allowable energy state of an atom Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Regents periodic table
Regents periodic table Electron configurations and periodicity
Electron configurations and periodicity Electronic configuration of mo (z=42)
Electronic configuration of mo (z=42) Atoms with 4 valence electrons
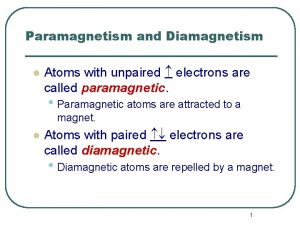
Atoms with 4 valence electrons Diamagnetic elements
Diamagnetic elements Arrangement of electrons in atoms chapter 4 test
Arrangement of electrons in atoms chapter 4 test Stable electron configurations are likely to contain
Stable electron configurations are likely to contain Chapter 5 review arrangement of electrons in atoms
Chapter 5 review arrangement of electrons in atoms Octet rule
Octet rule How do chemists model the valence electrons of metal atoms?
How do chemists model the valence electrons of metal atoms? Ionic and metallic bonding chapter 7 practice problems
Ionic and metallic bonding chapter 7 practice problems Copper subshell configuration
Copper subshell configuration 5 electrons in atoms
5 electrons in atoms Matterville answer key
Matterville answer key Periodic table refrence table
Periodic table refrence table Atom family song
Atom family song Perky patty proton
Perky patty proton The atoms family worksheet
The atoms family worksheet The atoms family atomic math challenge
The atoms family atomic math challenge What is periodicity
What is periodicity Texas health steps
Texas health steps Monophagic adalah
Monophagic adalah Chemsheets periodicity
Chemsheets periodicity Aap bright futures periodicity schedule
Aap bright futures periodicity schedule Atom size and electronegativity
Atom size and electronegativity Filariasis
Filariasis Relative formula mass of hcl
Relative formula mass of hcl Isotope abundance formula
Isotope abundance formula Differentiate between atomic number and mass number
Differentiate between atomic number and mass number Periodic table tends
Periodic table tends Trend of size in periodic table
Trend of size in periodic table Atomic number vs atomic radius
Atomic number vs atomic radius Quantum theory and the electronic structure of atoms
Quantum theory and the electronic structure of atoms Chapter 4 section 2 the structure of atoms answer key
Chapter 4 section 2 the structure of atoms answer key Chapter 6 electronic structure of atoms answers
Chapter 6 electronic structure of atoms answers Ap chemistry electronic structure of atoms
Ap chemistry electronic structure of atoms Which of the d orbitals most resembles a pz orbital?
Which of the d orbitals most resembles a pz orbital? Atomic structure and properties ap chemistry
Atomic structure and properties ap chemistry Atomic structure of periodic table
Atomic structure of periodic table Ape man chemistry
Ape man chemistry Interatomic bonding
Interatomic bonding