ATOMIC STRUCTURE AND PERIODIC TABLE TEST REVIEW CALCULATING
ATOMIC STRUCTURE AND PERIODIC TABLE TEST REVIEW

CALCULATING PROTONS, ELECTRONS, AND NEUTRONS • The number of protons and electrons will be the same as the atomic number. • The number of neutrons is found by rounding the atomic mass to the nearest whole number and then subtracting the atomic number.
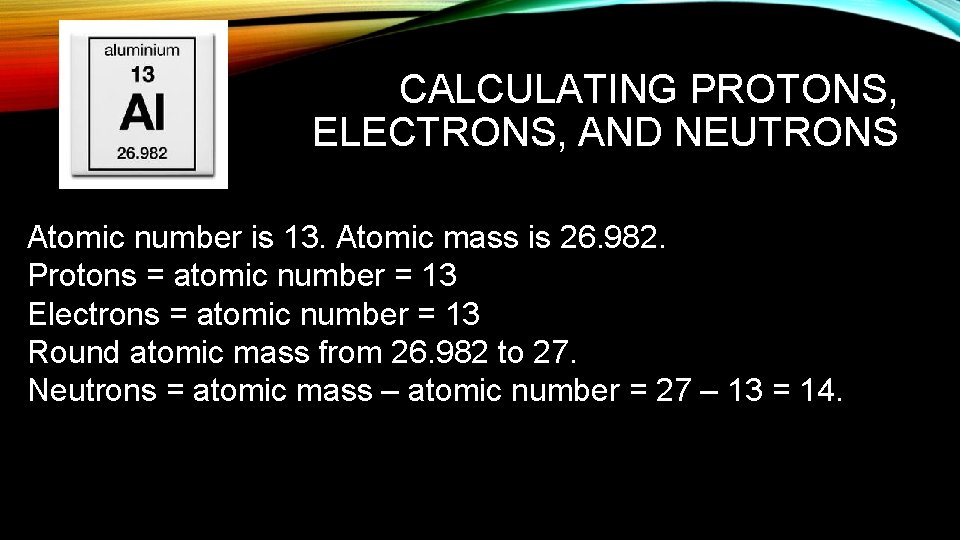
CALCULATING PROTONS, ELECTRONS, AND NEUTRONS Atomic number is 13. Atomic mass is 26. 982. Protons = atomic number = 13 Electrons = atomic number = 13 Round atomic mass from 26. 982 to 27. Neutrons = atomic mass – atomic number = 27 – 13 = 14.
CALCULATING PROTONS, ELECTRONS, AND NEUTRONS Calculate protons, electrons, and neutrons for Zinc – 67. Look up atomic number in periodic table. Atomic number is 30. Mass number is 67. Protons = atomic number = 30 Electrons = atomic number = 30 Neutrons = mass number – atomic number = 67 – 30 = 37.
ATOMIC NUMBER AND ATOMIC MASS • Atomic number is the number of protons. • Atomic mass is the number of protons plus neutrons. • For neutral atoms, the number of electrons is the same as the number of protons (or atomic number).

ISOTOPES • Isotopes have the same number of Protons • But a different number of Neutrons.
STRUCTURE OF ATOM • Protons and neutrons are in the nucleus. • Electrons are in the electron cloud surrounding the nucleus.

GROUPS: ALKALI METALS, ALKALINE EARTH METALS, TRANSITION METALS, CHALCOGENS, HALOGENS, NOBLE GASES • Potassium (#19) is in which group? • Alkali Metals • Gold (#79) is in which group? • Transition metals • Sulfur (#16) is in which group? • Chalcogens

BOHR MODEL • In the Bohr model, electrons are in Energy levels or shells.

RUTHERFORD’S GOLD FOIL EXPERIMENT • Most alpha particles went through foil because Atoms are mostly empty space • Some alpha particles bounced back because They were repelled by a concentration of positive charges (nucleus)

GROUP DESCRIPTIONS • Have incomplete inner electron shells • Good conductor • Malleable (can be pounded into shape) • Ductile (can be pulled into a wire) Transition metals

GROUP DESCRIPTIONS • Inert or chemically inactive • Do not bond with other elements Noble gases

GROUP DESCRIPTIONS • Low melting and boiling points • Very soft and can be cut with a knife Alkali metals

GROUP DESCRIPTIONS • These elements form salts. Halogens

ATOMIC SIZE • Which group (column) of the periodic table has the largest atoms? Group 1

MOST ABUNDANT ISOTOPE • What is the most abundant isotope of Krypton? Krypton – 84 What is the atomic mass of Krypton – 84? 84 amu What is the weighted atomic mass of Krypton? 83. 798 amu

USING PERIODIC TABLE • Element in period 2 group 14 Carbon • Element in period 6 group 16 Polonium • Element in period 5 group 4 Zirconium
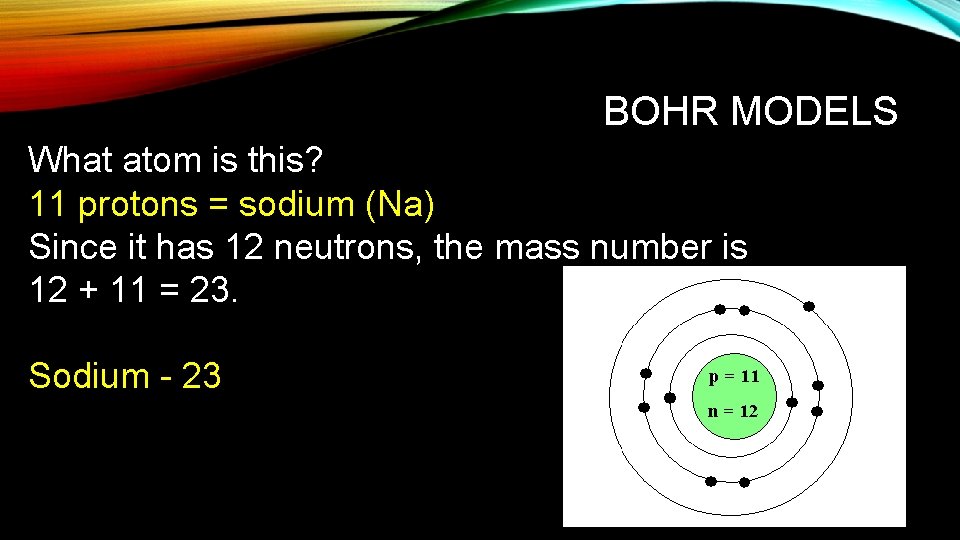
BOHR MODELS What atom is this? 11 protons = sodium (Na) Since it has 12 neutrons, the mass number is 12 + 11 = 23. Sodium - 23
MOST REACTIVE AND LEAST REACTIVE • Which groups are the most reactive? Alkali metals – Group 1 – most reactive metals Halogens – Group 17 – most reactive nonmetals • Which group is least reactive? Noble gases – Group 18
INFORMATION FOR TEST 1 Magnesium = Mg 2 Aluminum = Al • 4 Uranium = U • 9 a Sodium = Na b. Iridium = Ir c. Carbon = C d. Vanadium = V • 19 a Hydrogen = H b. Francium = Fr c. Fluorine = F d. Astatine = At • 20 Aluminum = Al • 23 Lithium = Li
- Slides: 20