Atomic Structure and Periodic Table 1 What are

Atomic Structure and Periodic Table 1
What are we going to learn ? n Part 1: Atomic structure n Historical background n n Inside the atom n n Dalton Thomson Rutherford/ Bohr Orbits and sub-orbits Atomic number and Atomic mass Electron configuration of elements Part 2: Periodic Table 2

Part 1 : Atomic structure Historical background 3

Dalton’s atomic theory n John Dalton, English scientist Matter is made up of atoms n All atoms of an element have the same mass and the same properties n Atoms are indestructible The main deficiency in Dalton’s theory n Atoms combine to form compounds was that the atom was considered indivisible. It could not explain concepts like charge, electromagnetism, radiation etc. n John Dalton 1766 - 1844 4
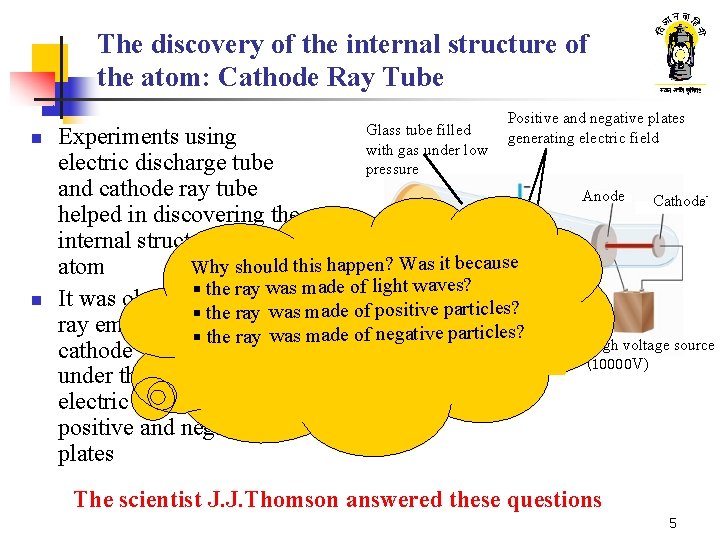
The discovery of the internal structure of the atom: Cathode Ray Tube n n Glass tube filled with gas under low pressure Positive and negative plates generating electric field Experiments using electric discharge tube and cathode ray tube helped in discovering the internal structure of an Why should this happen? Was it because atom the ray was made of light waves? § It was observed that the made of positive particles? § the ray was ray emanating§from the ray was made of negative particles? cathode would deviate Fluroscent under the influence of screen electric field between the positive and negative plates Anode Cathodeë High voltage source (10000 V) The scientist J. J. Thomson answered these questions 5
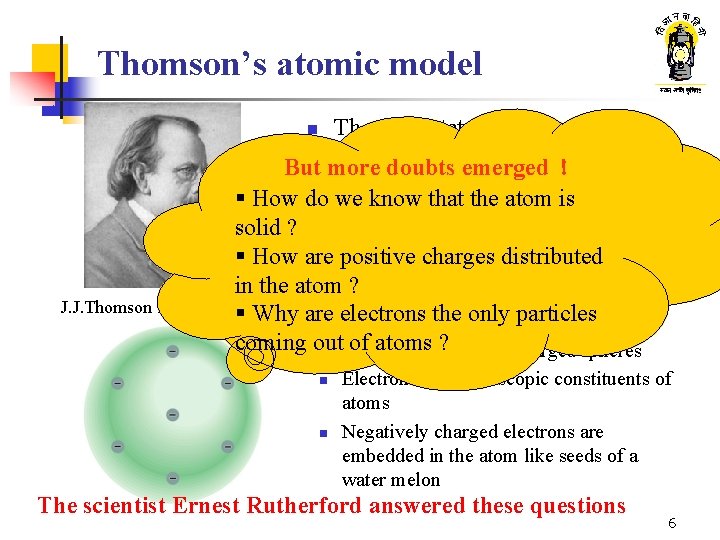
Thomson’s atomic model Thomson stated that n n Cathode rays are a stream of particles But more doubts emerged ! (electrons) ; not light rays § How do we know that the atom is n Considering their deviation these solid ? particles must be negatively charged. § How are positive charges distributed in the atom ? J. J. Thomson 1856 - 1940§ Why are electrons the only particles n Thomson’s atomic model coming out of atoms ? n Atoms are positively charged spheres n n Electrons are microscopic constituents of atoms Negatively charged electrons are embedded in the atom like seeds of a water melon The scientist Ernest Rutherford answered these questions 6
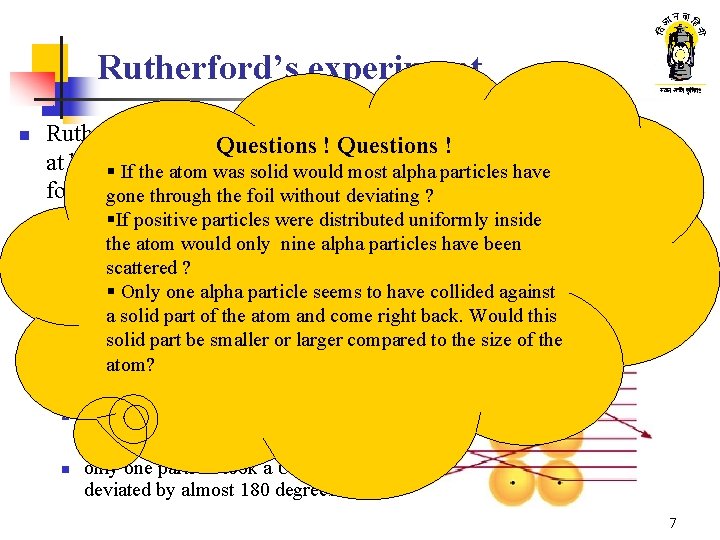
Rutherford’s experiment Thin gold foil n Alpha ray Rutherford fired alpha particles Questions ! Polonium at high§ velocity a thin If the atomon was solid gold would most alpha particles have foil gone through the foil without deviating ? §If particles: positive particles Alpha Minute were distributed uniformly inside the atom wouldparticles. only nine alpha particles have been positively charged Alpha particle detector scattered Their source ? : Polonium Box lined with Lead screen 1/ § Only of onegold alpha seems to have collided against n Thickness foilparticle 50000 cm a solid part of the atom and come right back. Would this Out ofsolid approx. particles part be 20000 smalleralpha or larger compared to the size of the n about 19990 passed through the foil atom? without deviation ; n n nine particles were scattered in various directions; only one particle took a U turn and deviated by almost 180 degrees 7
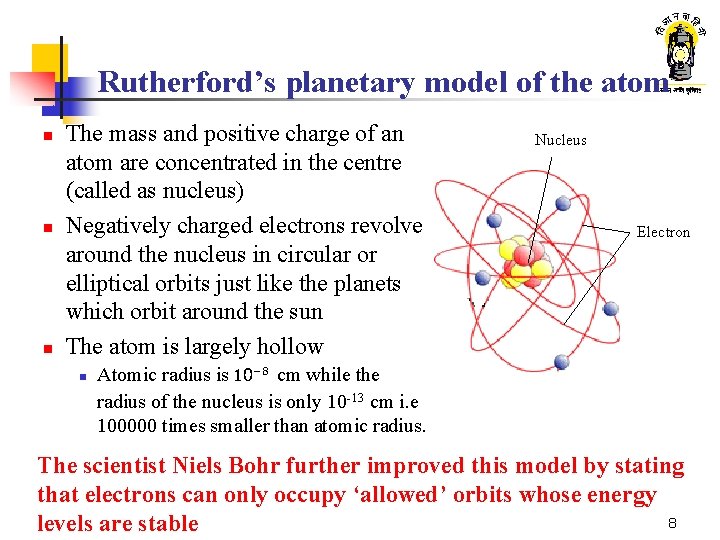
Rutherford’s planetary model of the atom n n n The mass and positive charge of an atom are concentrated in the centre (called as nucleus) Negatively charged electrons revolve around the nucleus in circular or elliptical orbits just like the planets which orbit around the sun The atom is largely hollow n Nucleus Electron Atomic radius is 10 -8 cm while the radius of the nucleus is only 10 -13 cm i. e 100000 times smaller than atomic radius. The scientist Niels Bohr further improved this model by stating that electrons can only occupy ‘allowed’ orbits whose energy 8 levels are stable
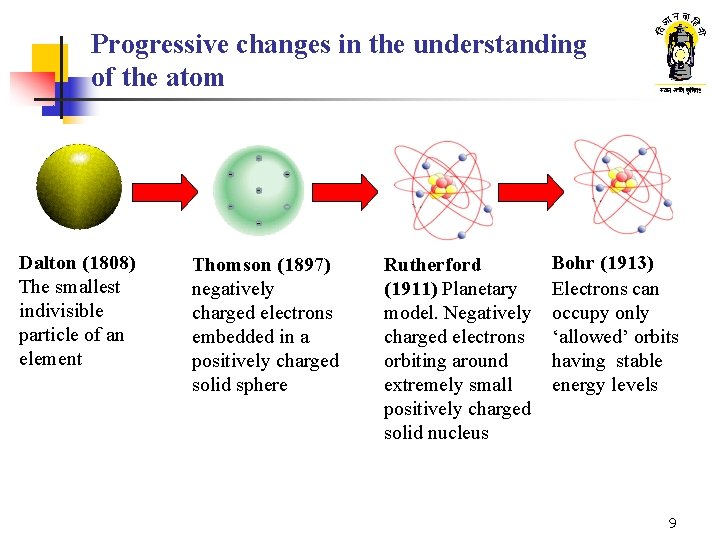
Progressive changes in the understanding of the atom Dalton (1808) The smallest indivisible particle of an element Thomson (1897) negatively charged electrons embedded in a positively charged solid sphere Rutherford (1911) Planetary model. Negatively charged electrons orbiting around extremely small positively charged solid nucleus Bohr (1913) Electrons can occupy only ‘allowed’ orbits having stable energy levels 9

Part 1: Atomic structure (contd) Inside the atom 10
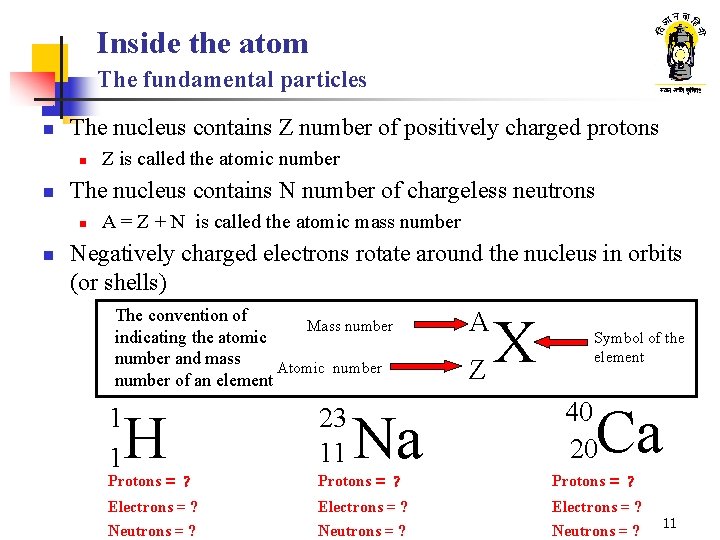
Inside the atom The fundamental particles n The nucleus contains Z number of positively charged protons n n The nucleus contains N number of chargeless neutrons n n Z is called the atomic number A = Z + N is called the atomic mass number Negatively charged electrons rotate around the nucleus in orbits (or shells) The convention of Mass number indicating the atomic number and mass Atomic number of an element 1 1 H 23 11 Na A X Z Symbol of the element 40 20 Ca Protons = ? Electrons = ? Neutrons = ? 11
Inside the atom Fundamental particles n n n The attraction between positively charged nucleus and negatively charged electrons keeps the electrons within the atom An orbit with orbit number ‘n’ can contain maximum 2 n 2 electrons In neutral atoms the number of protons is equal to the number of electrons Protons and neutrons have the same mass (1. 6 x 10– 24 gm) The mass of an electron is 1837 times less than that of a proton The chemical properties of an element depend upon the electrons in the outermost orbit 12
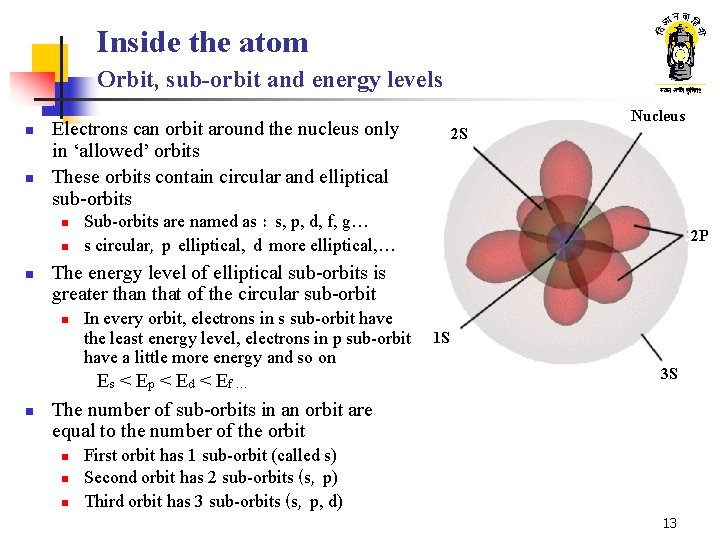
Inside the atom Orbit, sub-orbit and energy levels n n n 2 S Sub-orbits are named as : s, p, d, f, g… s circular, p elliptical, d more elliptical, … 2 P The energy level of elliptical sub-orbits is greater than that of the circular sub-orbit n n Nucleus Electrons can orbit around the nucleus only in ‘allowed’ orbits These orbits contain circular and elliptical sub-orbits In every orbit, electrons in s sub-orbit have the least energy level, electrons in p sub-orbit have a little more energy and so on Es < E p < E d < E f … The number of sub-orbits in an orbit are equal to the number of the orbit n n n 1 S 3 S First orbit has 1 sub-orbit (called s) Second orbit has 2 sub-orbits (s, p) Third orbit has 3 sub-orbits (s, p, d) 13
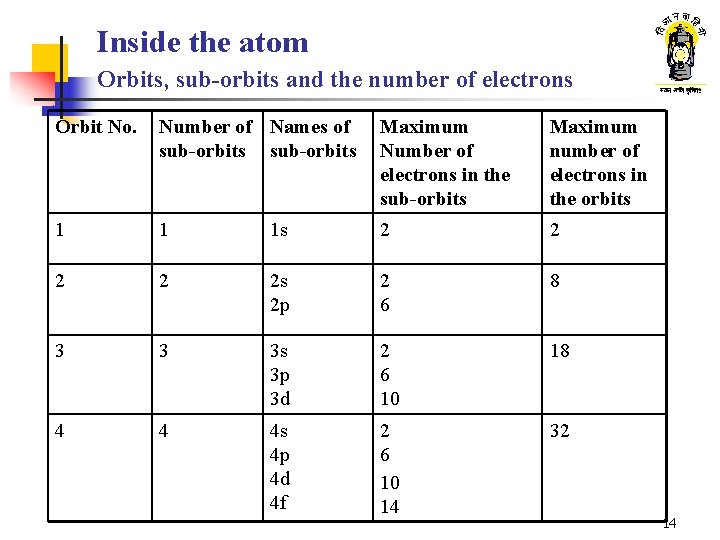
Inside the atom Orbits, sub-orbits and the number of electrons Orbit No. Number of Names of sub-orbits Maximum Number of electrons in the sub-orbits Maximum number of electrons in the orbits 1 1 1 s 2 2 2 s 2 p 2 6 8 3 3 3 s 3 p 3 d 2 6 10 18 4 4 4 s 4 p 4 d 4 f 2 6 10 14 32 14
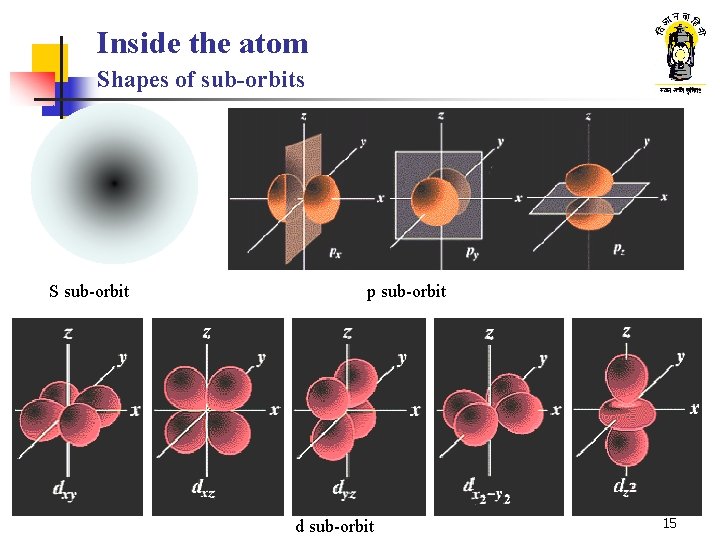
Inside the atom Shapes of sub-orbits S sub-orbit p sub-orbit d sub-orbit 15
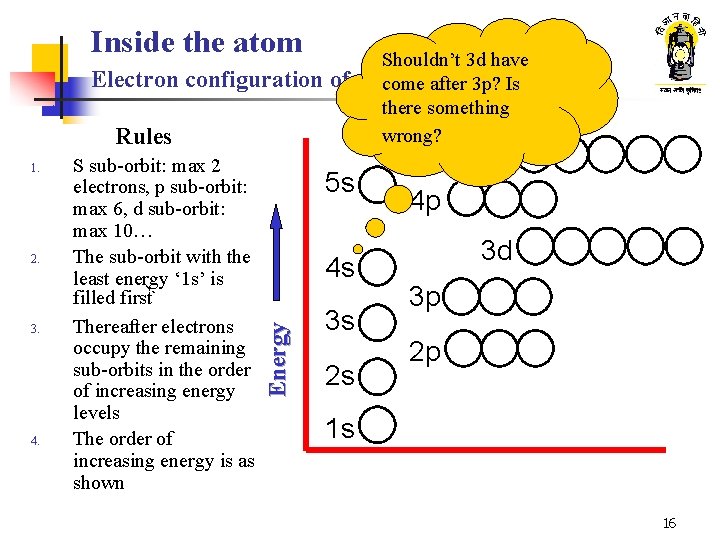
Inside the atom Electron configuration of Rules 2. 3. 4. S sub-orbit: max 2 electrons, p sub-orbit: max 6, d sub-orbit: max 10… The sub-orbit with the least energy ‘ 1 s’ is filled firstå Thereafter electrons occupy the remaining sub-orbits in the order of increasing energy levels The order of increasing energy is as shown 5 s 4 s Energy 1. 3 s 2 s Shouldn’t 3 d have elements come after 3 p? Is there something wrong? 4 d 4 p 3 d 3 p 2 p 1 s 16
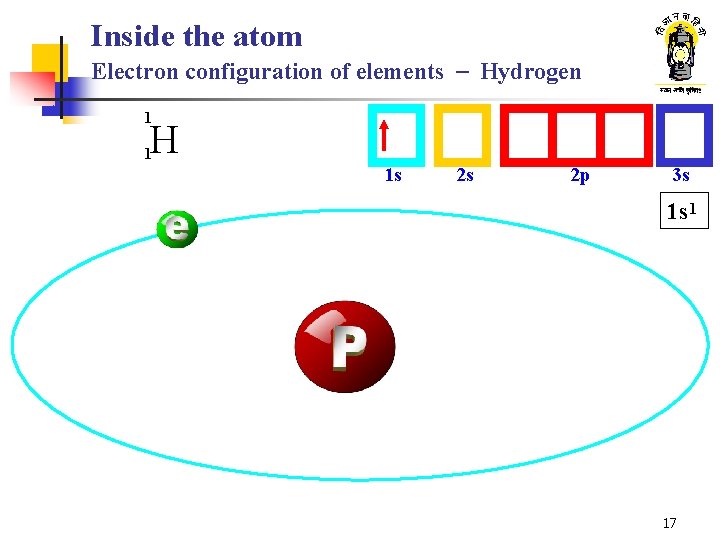
Inside the atom Electron configuration of elements - Hydrogen 1 H 1 1 s 2 s 2 p 3 s 1 s 1 17
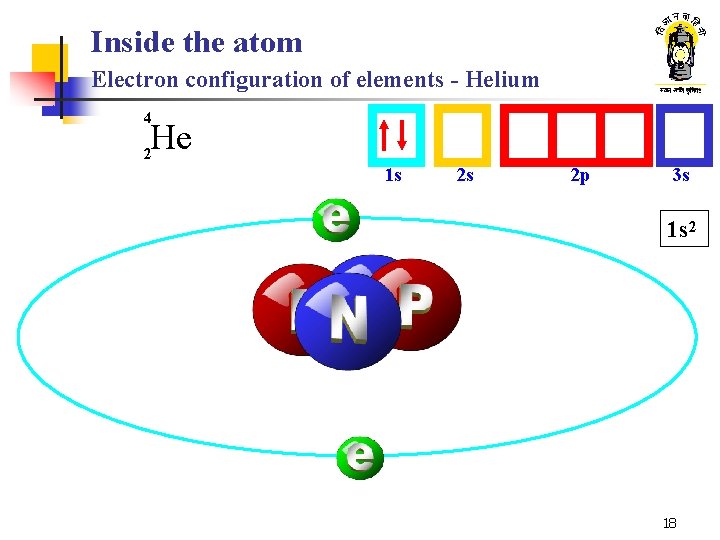
Inside the atom Electron configuration of elements - Helium 4 He 2 1 s 2 s 2 p 3 s 1 s 2 18
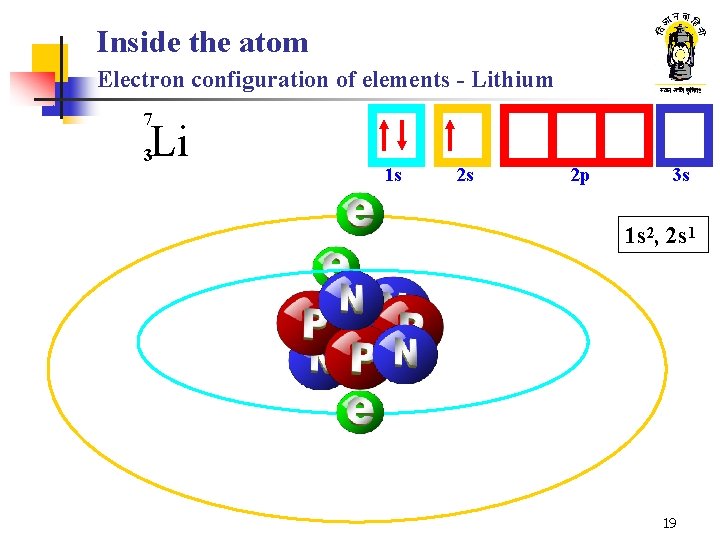
Inside the atom Electron configuration of elements - Lithium 7 Li 3 1 s 2 s 2 p 3 s 1 s 2, 2 s 1 19
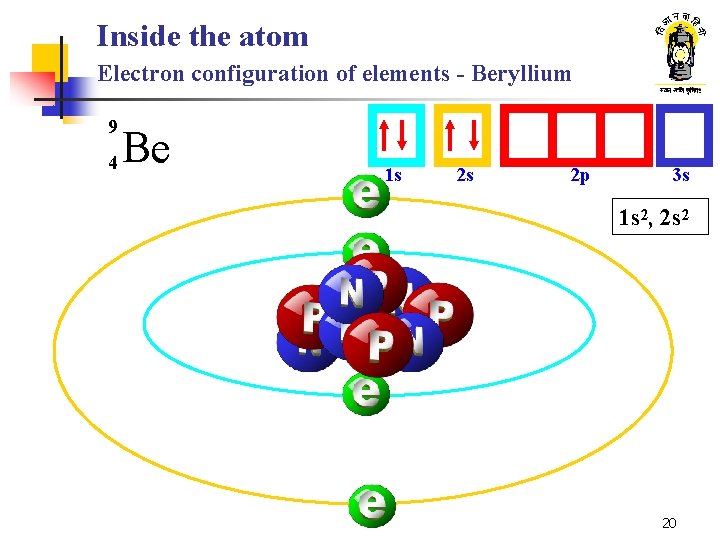
Inside the atom Electron configuration of elements - Beryllium 9 4 Be 1 s 2 s 2 p 3 s 1 s 2, 2 s 2 20
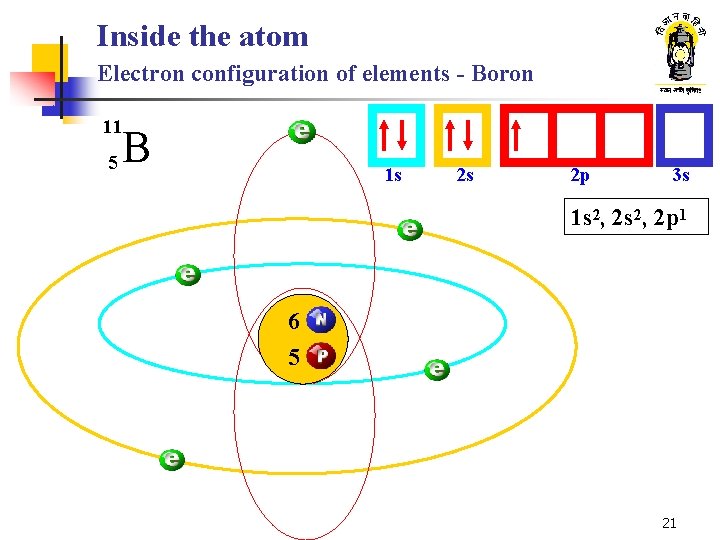
Inside the atom Electron configuration of elements - Boron 11 5 B 1 s 2 s 2 p 3 s 1 s 2, 2 p 1 6 5 21
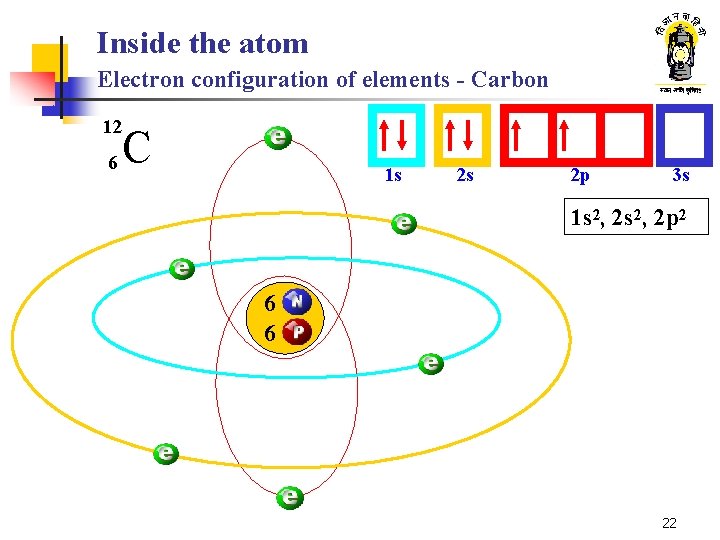
Inside the atom Electron configuration of elements - Carbon 12 6 C 1 s 2 s 2 p 3 s 1 s 2, 2 p 2 6 6 22
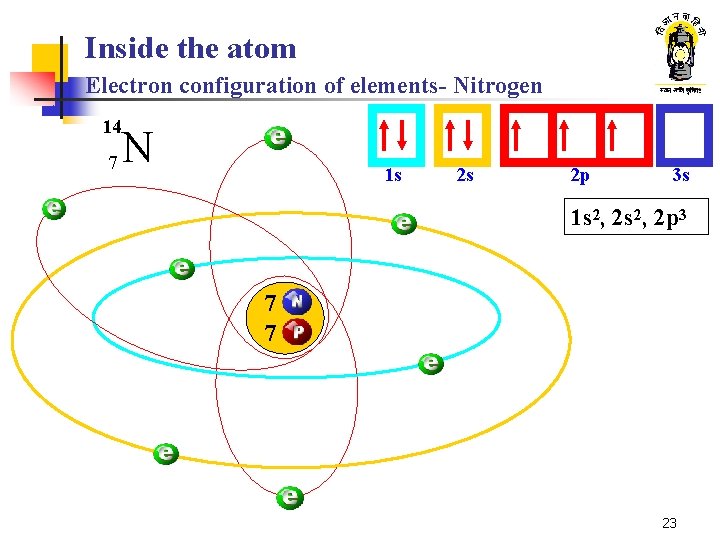
Inside the atom Electron configuration of elements- Nitrogen 14 7 N 1 s 2 s 2 p 3 s 1 s 2, 2 p 3 7 7 23
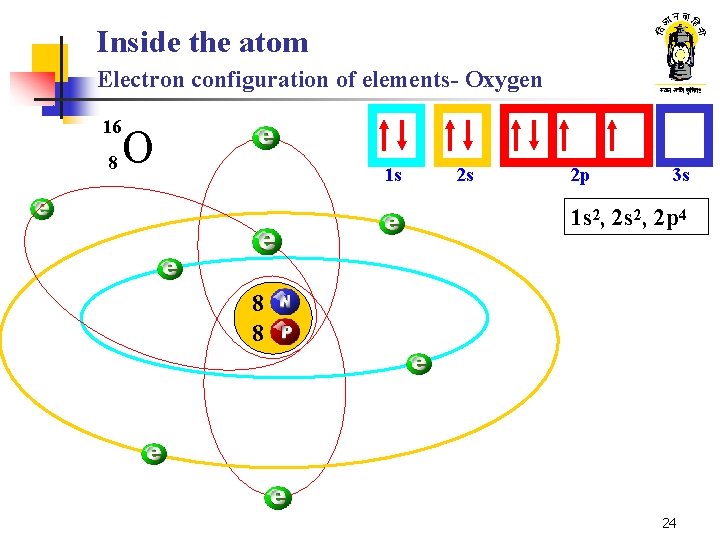
Inside the atom Electron configuration of elements- Oxygen 16 8 O 1 s 2 s 2 p 3 s 1 s 2, 2 p 4 8 8 24
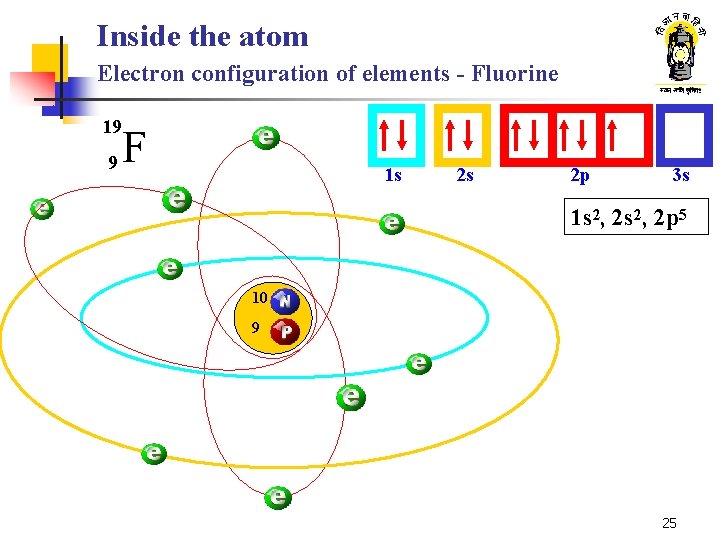
Inside the atom Electron configuration of elements - Fluorine 19 9 F 1 s 2 s 2 p 3 s 1 s 2, 2 p 5 10 9 25
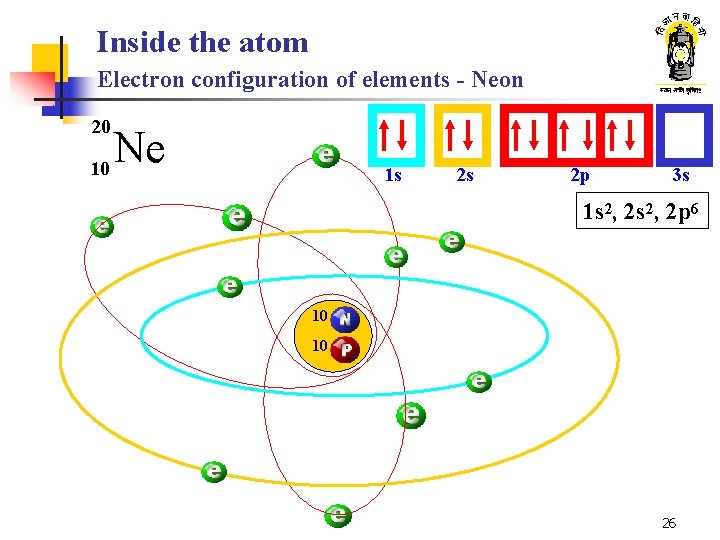
Inside the atom Electron configuration of elements - Neon 20 10 Ne 1 s 2 s 2 p 3 s 1 s 2, 2 p 6 10 10 26
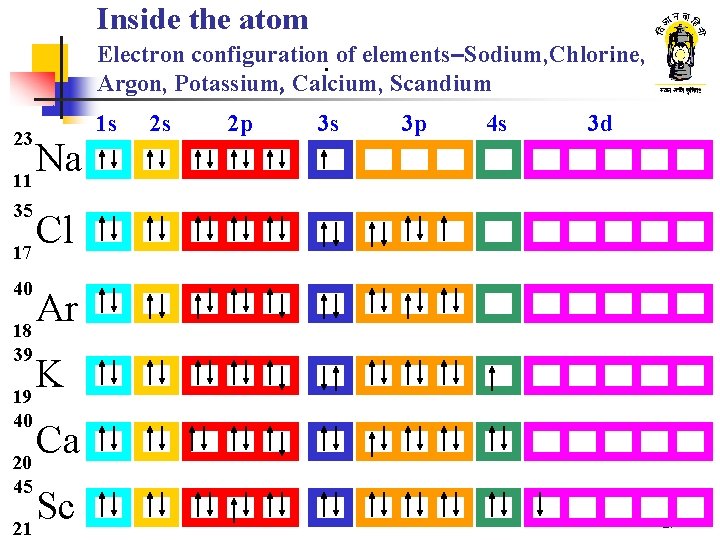
Inside the atom. Electron configuration. of elements–Sodium, Chlorine, Argon, Potassium, Calcium, Scandium 23 Na 11 1 s 2 s 2 p 3 s 3 p 4 s 3 d 35 Cl 17 40 Ar 18 39 K 19 40 Ca 20 45 Sc 21 27
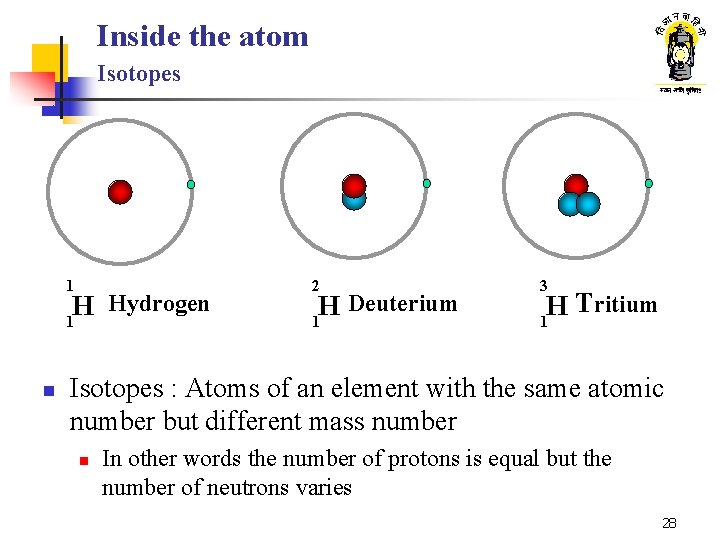
Inside the atom Isotopes 1 Hydrogen H 1 n 2 Deuterium H 1 3 Tritium H 1 Isotopes : Atoms of an element with the same atomic number but different mass number n In other words the number of protons is equal but the number of neutrons varies 28
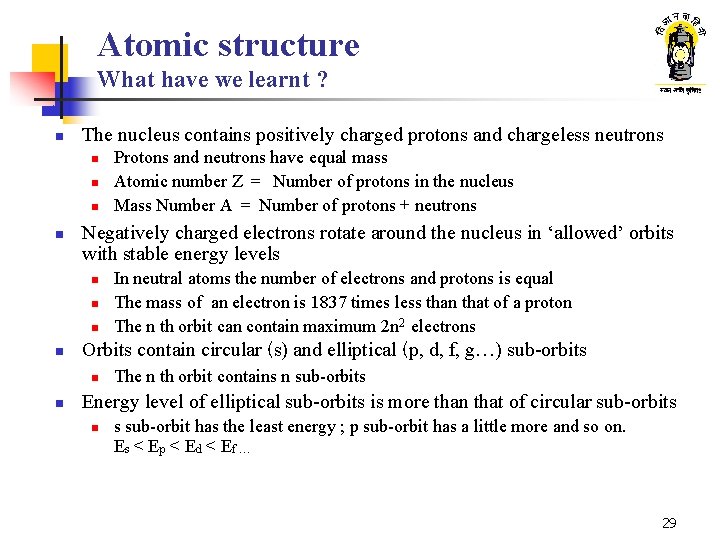
Atomic structure What have we learnt ? n The nucleus contains positively charged protons and chargeless neutrons n n Negatively charged electrons rotate around the nucleus in ‘allowed’ orbits with stable energy levels n n In neutral atoms the number of electrons and protons is equal The mass of an electron is 1837 times less than that of a proton The n th orbit can contain maximum 2 n 2 electrons Orbits contain circular (s) and elliptical (p, d, f, g…) sub-orbits n n Protons and neutrons have equal mass Atomic number Z = Number of protons in the nucleus Mass Number A = Number of protons + neutrons The n th orbit contains n sub-orbits Energy level of elliptical sub-orbits is more than that of circular sub-orbits n s sub-orbit has the least energy ; p sub-orbit has a little more and so on. Es < E p < E d < E f … 29
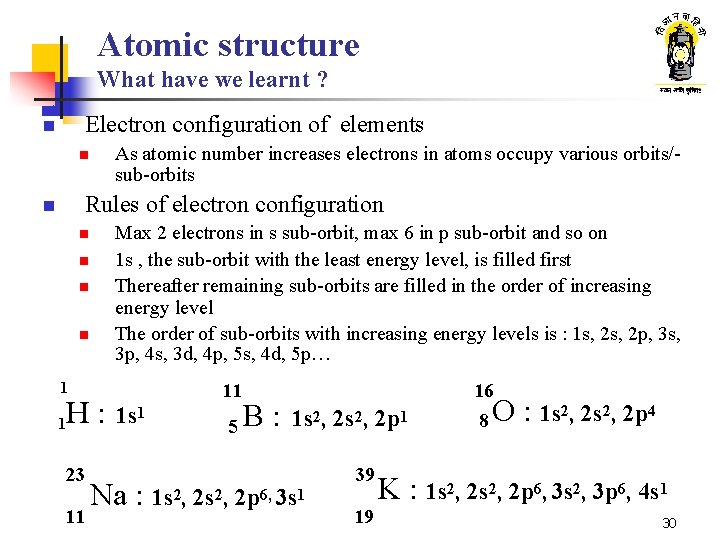
Atomic structure What have we learnt ? Electron configuration of elements n n As atomic number increases electrons in atoms occupy various orbits/sub-orbits Rules of electron configuration n n Max 2 electrons in s sub-orbit, max 6 in p sub-orbit and so on 1 s , the sub-orbit with the least energy level, is filled first Thereafter remaining sub-orbits are filled in the order of increasing energy level The order of sub-orbits with increasing energy levels is : 1 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, 5 s, 4 d, 5 p… 1 1 H: 23 11 1 s 1 11 5 B : 16 1 s 2, 2 p 1 Na : 1 s 2, 2 p 6, 3 s 1 39 19 8 O : 1 s 2, 2 p 4 K : 1 s 2, 2 p 6, 3 s 2, 3 p 6, 4 s 1 30
Part 2 Periodic table Based on our knowledge of electron configuration of elements we shall see how the elements can be logically ordered 31
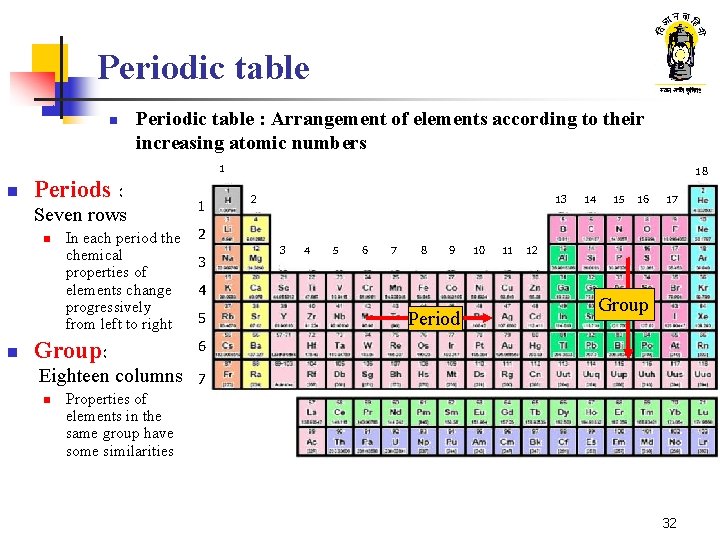
Periodic table n Periodic table : Arrangement of elements according to their increasing atomic numbers 1 n Periods : Seven rows n n In each period the chemical properties of elements change progressively from left to right Group: Eighteen columns n 1 18 2 13 14 15 16 17 2 3 3 4 5 6 7 8 9 4 5 Period 10 11 12 Group 6 7 Properties of elements in the same group have some similarities 32
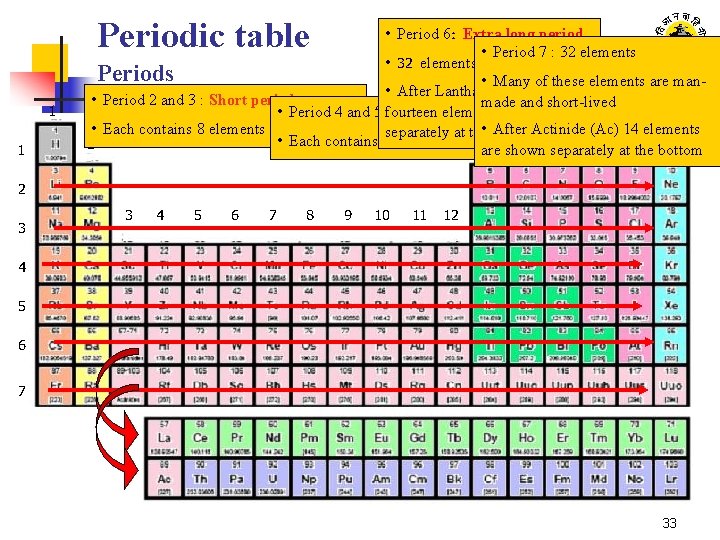
Periodic table 1 1 • Period 6: Extra long period • Period 7 : 32 elements • 32 elements Periods • Many of these elements are man • After Lanthanum (La) • Period 2 and 3 : Short periods made and short-lived 18 • Period 4 and 5 fourteen : Long periods elements are shown • Each contains 8 elements After Actinide (Ac) 14 elements separately at the • bottom • Each contains 18 elements 2 13 shown 14 separately 15 16 at 17 the bottom are 2 3 3 4 5 6 7 8 9 10 11 12 4 5 6 7 33
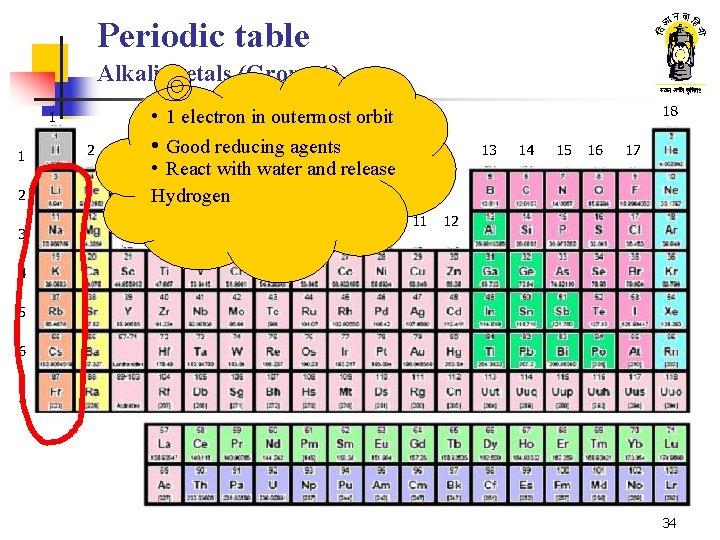
Periodic table Alkali metals (Group 1) 1 2 2 3 18 • 1 electron in outermost orbit • Good reducing agents • React with water and release Hydrogen 1 3 4 5 6 7 8 9 10 13 11 14 15 16 17 12 4 5 6 7 34
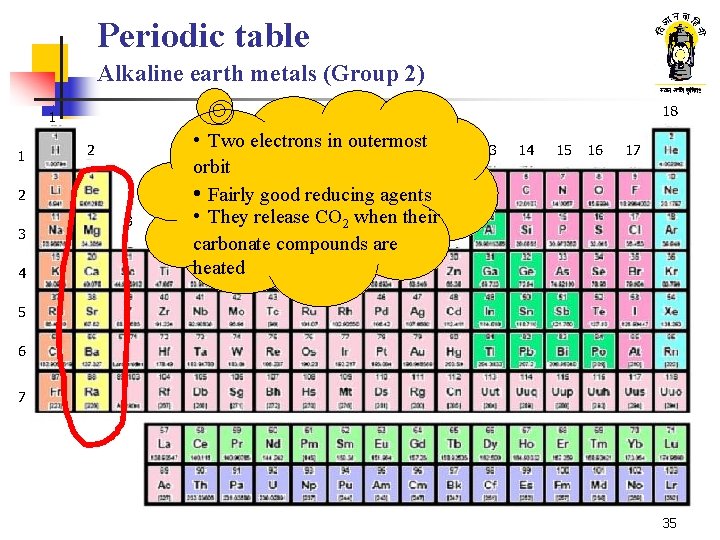
Periodic table Alkaline earth metals (Group 2) 18 1 1 2 2 3 4 • Two electrons in outermost orbit • Fairly good reducing agents • 5 They 6 release 7 8 CO 29 when 10 their 11 12 carbonate compounds are heated 13 14 15 16 17 5 6 7 35
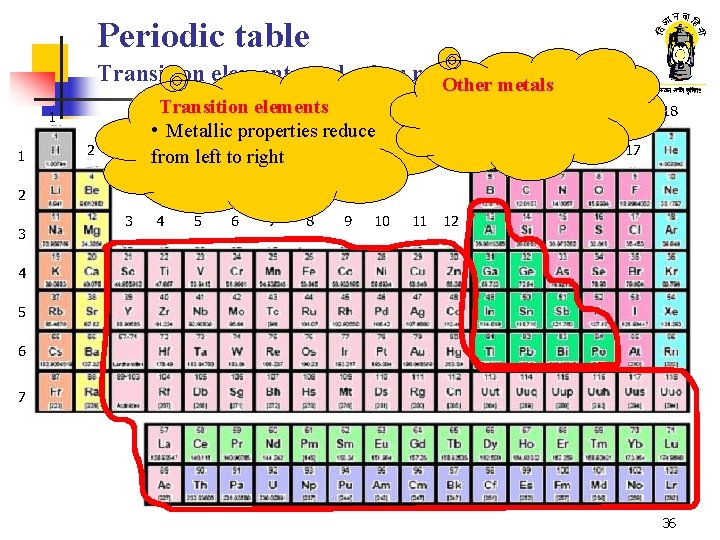
Periodic table Transition elements and other metals Other metals Transition elements • Metallic properties reduce from left to right 1 1 2 18 13 14 15 16 17 2 3 3 4 5 6 7 8 9 10 11 12 4 5 6 7 36
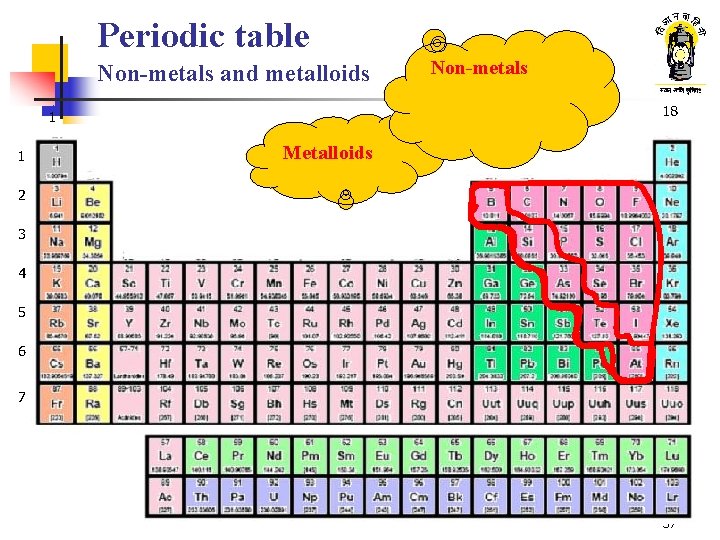
Periodic table Non-metals and metalloids 18 1 1 2 13 Metalloids 14 15 16 17 2 3 3 4 5 6 7 8 9 10 11 12 4 5 6 7 37
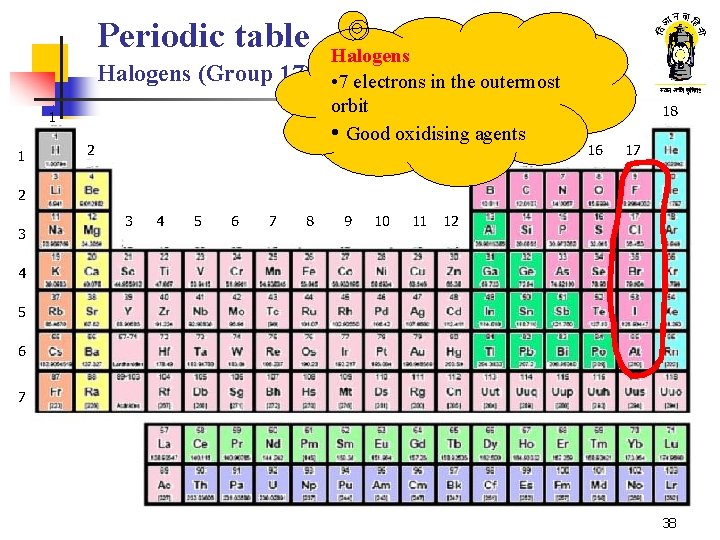
Periodic table Halogens (Group 17) 1 1 2 Halogens • 7 electrons in the outermost orbit • Good oxidising agents 13 14 15 18 16 17 2 3 3 4 5 6 7 8 9 10 11 12 4 5 6 7 38
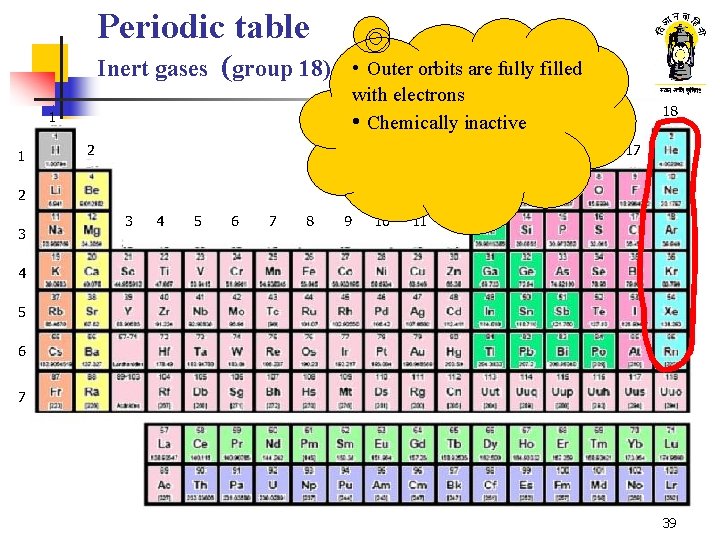
Periodic table Inert gases (group 18) • Outer orbits are fully filled with electrons • Chemically inactive 1 1 2 13 14 18 15 16 17 2 3 3 4 5 6 7 8 9 10 11 12 4 5 6 7 39
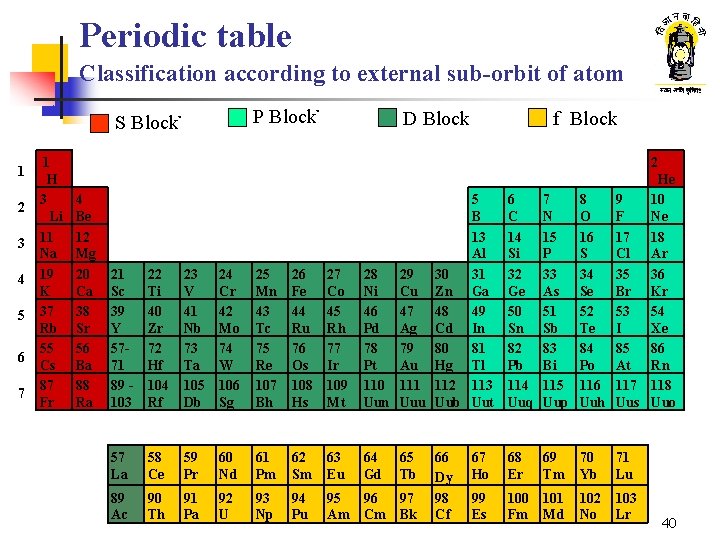
Periodic table Classification according to external sub-orbit of atom P Blockû S Blockû 1 2 3 4 5 6 7 1 H 3 Li 11 Na 19 K 37 Rb 55 Cs 87 Fr D Block f Block 2 4 Be 12 Mg 20 Ca 38 Sr 56 Ba 88 Ra 21 Sc 39 Y 5771 89 103 22 Ti 40 Zr 72 Hf 104 Rf 23 V 41 Nb 73 Ta 105 Db 24 Cr 42 Mo 74 W 106 Sg 25 Mn 43 Tc 75 Re 107 Bh 26 Fe 44 Ru 76 Os 108 Hs 27 Co 45 Rh 77 Ir 109 Mt He 5 6 7 8 9 10 B C N O F Ne 13 14 15 16 17 18 Al Si P S Cl Ar 28 29 30 31 32 33 34 35 36 Ni Cu Zn Ga Ge As Se Br Kr 46 47 48 49 50 51 52 53 54 Pd Ag Cd In Sn Sb Te I Xe 78 79 80 81 82 83 84 85 86 Pt Au Hg Tl Pb Bi Po At Rn 110 111 112 113 114 115 116 117 118 Uun Uuu Uub Uut Uuq Uup Uuh Uus Uuo 57 La 58 Ce 59 Pr 60 Nd 61 Pm 62 Sm 63 Eu 64 Gd 65 Tb 89 Ac 90 Th 91 Pa 92 U 93 Np 94 Pu 95 Am 96 Cm 97 Bk 66 Dy 98 Cf 67 Ho 68 Er 69 Tm 70 Yb 71 Lu 99 Es 100 Fm 101 Md 102 No 103 Lr 40
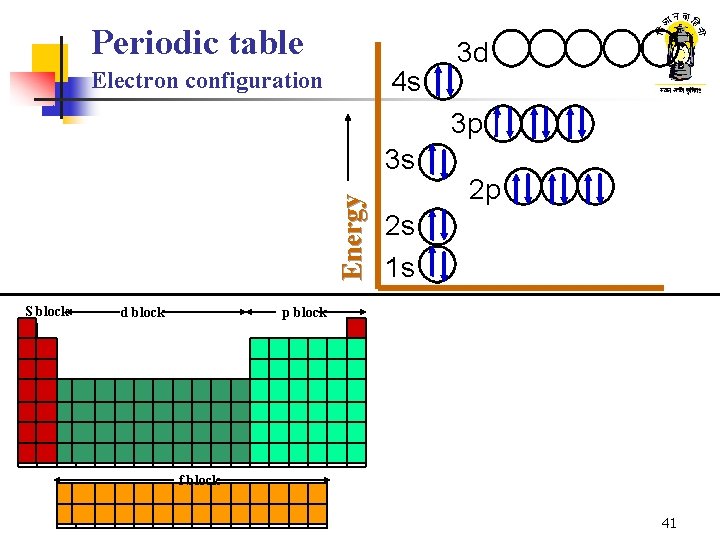
Periodic table 4 s Electron configuration 3 d 3 p Energy 3 s S block d block He Be B C N O F Ne Na Mg K 2 s 1 s p block H Li 2 p Al Ca Sc Ti V Rb Sr Cs Ba Si P S Cl Ar Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Hf Ta W Re Os Ir Xe Pt Au Hg Tl Pb Bi Po At Rn Fr Ra f block La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Ac Th Pa U 41
Periodic table: What have we learnt ? n n n Periodic table : Arrangement of elements in the order of their atomic number Contains 18 columns (called ‘Groups’) and 7 rows (called ‘Periods’) Elements in the same group have similar chemical properties n n n First group: Alkali metals (Good reducing agents) Second group: Alkaline earth metals (Fairly good reducing agent) Seventeenth group: Halogens (Good oxidising agents) Eighteenth group : Inert gases In a period, as you go from left to right, metallic properties reduce while non-metallic properties increase å n n n Second and third period : Short periods (8 elements) Fourth and fifth periods : Long periods (18 elements) Sixth and seventh period : Extra long period (32 elements) 42

The End 43
- Slides: 43