ATOMIC STRUCTURE An Atom is the smallest unit
- Slides: 13

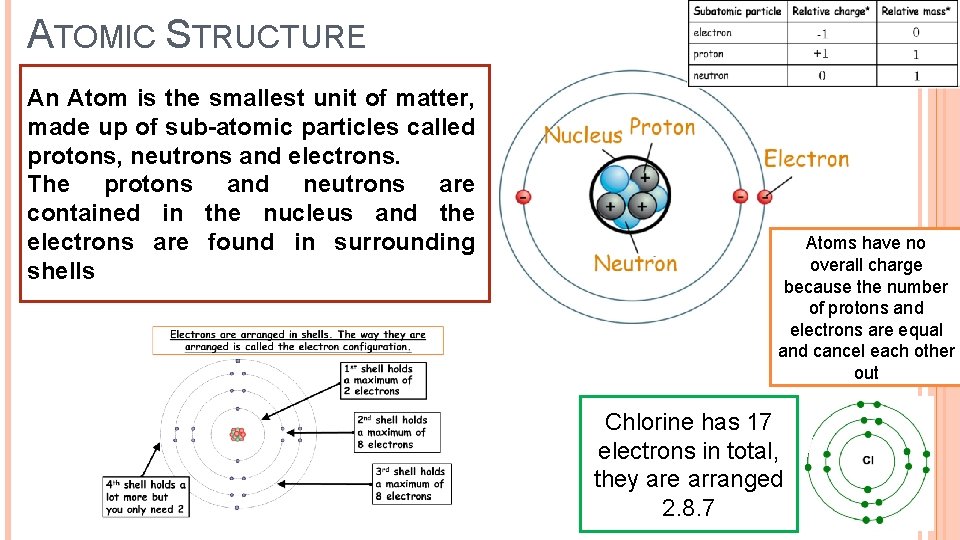
ATOMIC STRUCTURE An Atom is the smallest unit of matter, made up of sub-atomic particles called protons, neutrons and electrons. The protons and neutrons are contained in the nucleus and the electrons are found in surrounding shells Atoms have no overall charge because the number of protons and electrons are equal and cancel each other out Chlorine has 17 electrons in total, they are arranged 2. 8. 7

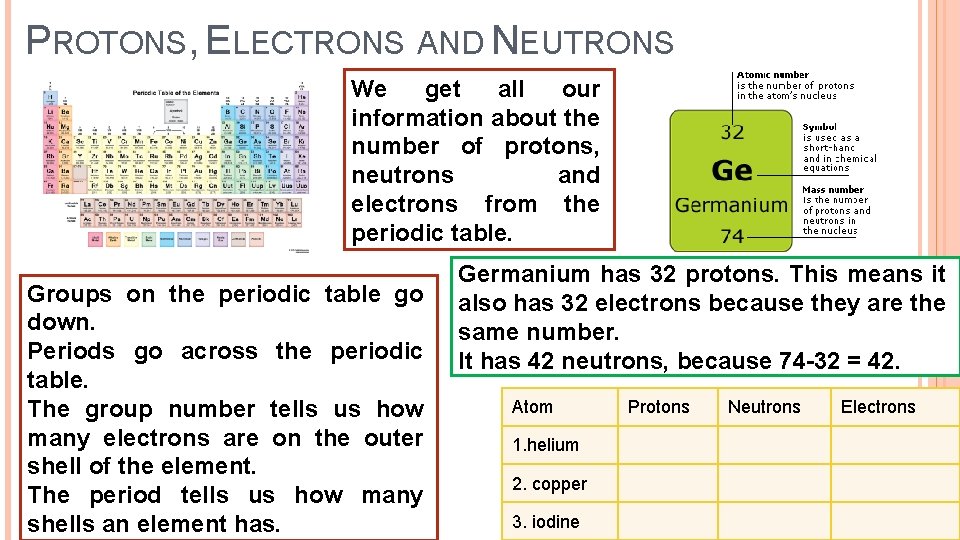
PROTONS, ELECTRONS AND NEUTRONS We get all our information about the number of protons, neutrons and electrons from the periodic table. Groups on the periodic table go down. Periods go across the periodic table. The group number tells us how many electrons are on the outer shell of the element. The period tells us how many shells an element has. Germanium has 32 protons. This means it also has 32 electrons because they are the same number. It has 42 neutrons, because 74 -32 = 42. Atom 1. helium 2. copper 3. iodine Protons Neutrons Electrons

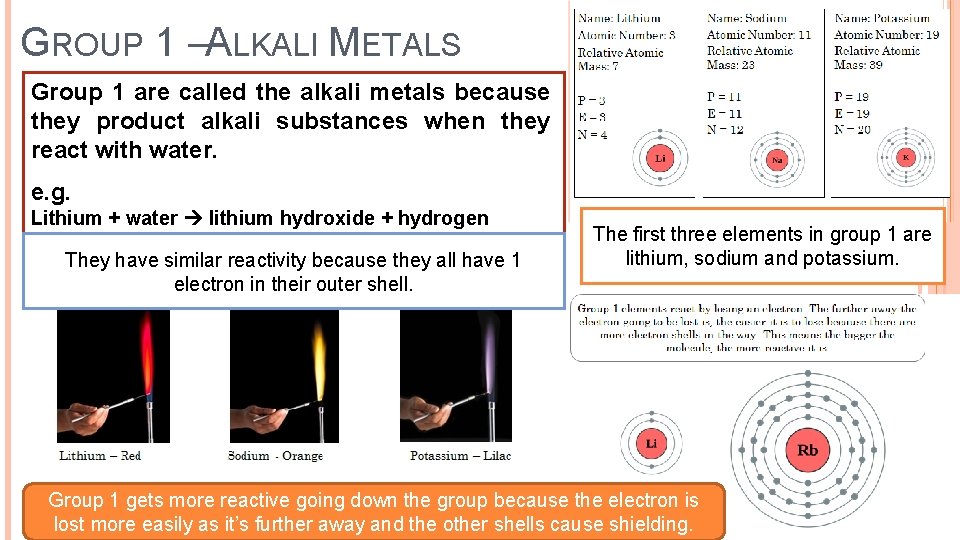
GROUP 1 –ALKALI METALS Group 1 are called the alkali metals because they product alkali substances when they react with water. e. g. Lithium + water lithium hydroxide + hydrogen They have similar reactivity because they all have 1 electron in their outer shell. The first three elements in group 1 are lithium, sodium and potassium. Group 1 gets more reactive going down the group because the electron is lost more easily as it’s further away and the other shells cause shielding.

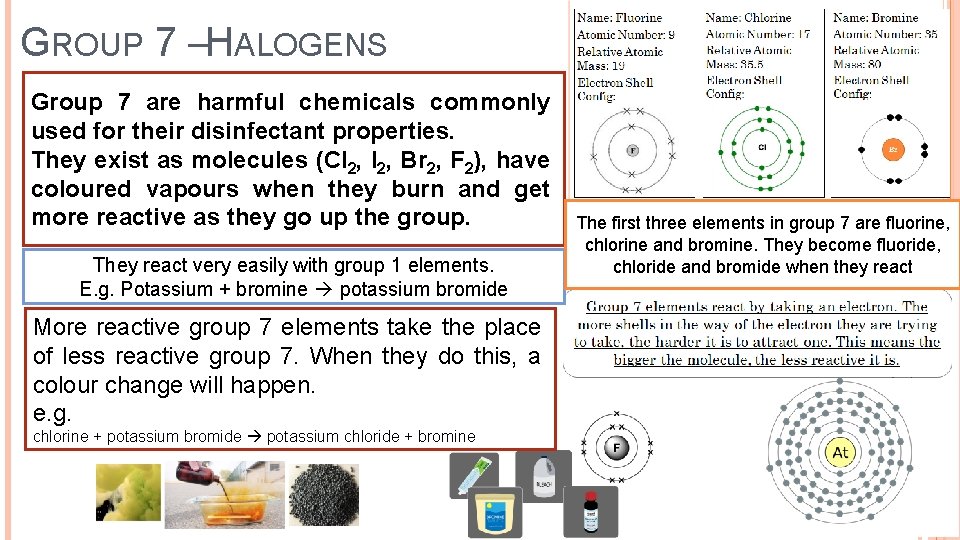
GROUP 7 –HALOGENS Group 7 are harmful chemicals commonly used for their disinfectant properties. They exist as molecules (Cl 2, I 2, Br 2, F 2), have coloured vapours when they burn and get more reactive as they go up the group. They react very easily with group 1 elements. E. g. Potassium + bromine potassium bromide More reactive group 7 elements take the place of less reactive group 7. When they do this, a colour change will happen. e. g. chlorine + potassium bromide potassium chloride + bromine The first three elements in group 7 are fluorine, chlorine and bromine. They become fluoride, chloride and bromide when they react

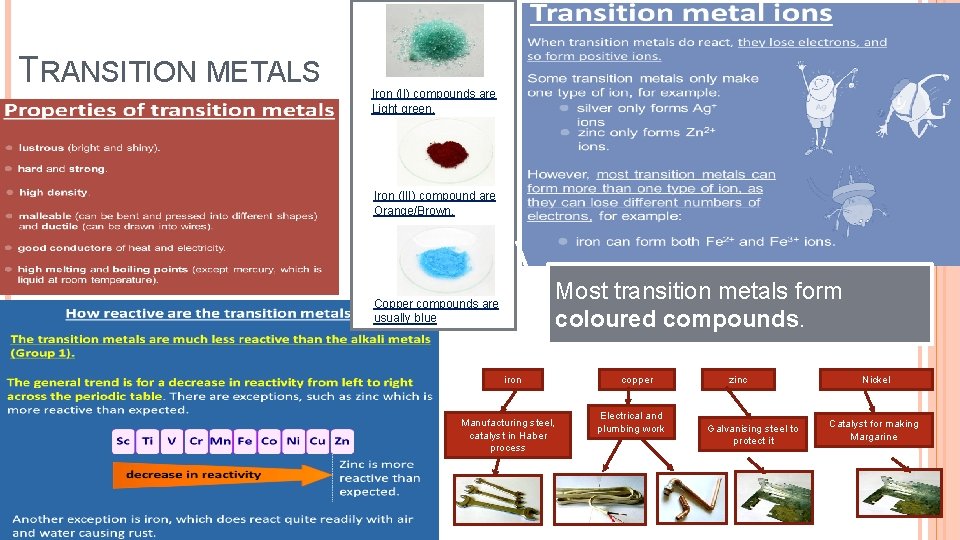
TRANSITION METALS Iron (II) compounds are Light green. Iron (III) compound are Orange/Brown. Most transition metals form coloured compounds. Copper compounds are usually blue iron Manufacturing steel, catalyst in Haber process copper Electrical and plumbing work zinc Galvanising steel to protect it Nickel Catalyst for making Margarine

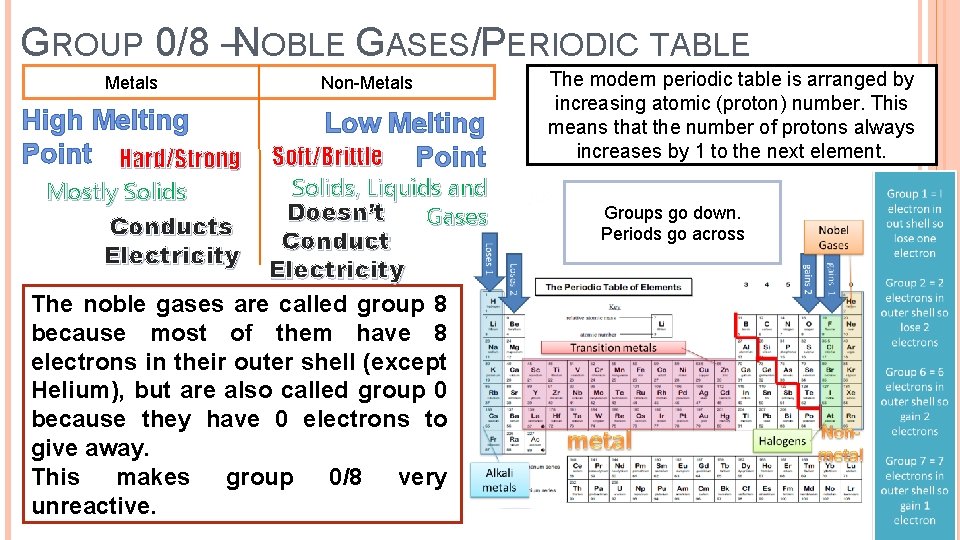
GROUP 0/8 –NOBLE GASES/PERIODIC TABLE Metals High Melting Point Hard/Strong Non-Metals Low Melting Soft/Brittle Point Solids, Liquids and Doesn’t Gases Conduct Electricity The noble gases are called group 8 because most of them have 8 electrons in their outer shell (except Helium), but are also called group 0 because they have 0 electrons to give away. This makes group 0/8 very unreactive. Mostly Solids Conducts Electricity The modern periodic table is arranged by increasing atomic (proton) number. This means that the number of protons always increases by 1 to the next element. Groups go down. Periods go across

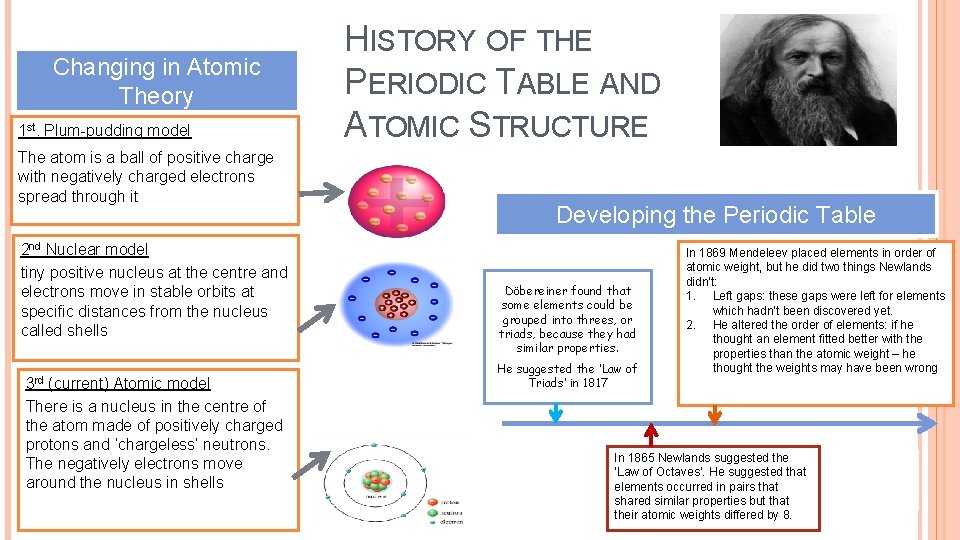
Changing in Atomic Theory 1 st. Plum-pudding model The atom is a ball of positive charge with negatively charged electrons spread through it 2 nd Nuclear model tiny positive nucleus at the centre and electrons move in stable orbits at specific distances from the nucleus called shells 3 rd (current) Atomic model There is a nucleus in the centre of the atom made of positively charged protons and ‘chargeless’ neutrons. The negatively electrons move around the nucleus in shells HISTORY OF THE PERIODIC TABLE AND ATOMIC STRUCTURE Developing the Periodic Table Döbereiner found that some elements could be grouped into threes, or triads, because they had similar properties. He suggested the ‘Law of Triads’ in 1817 In 1869 Mendeleev placed elements in order of atomic weight, but he did two things Newlands didn’t: 1. Left gaps: these gaps were left for elements which hadn’t been discovered yet. 2. He altered the order of elements: if he thought an element fitted better with the properties than the atomic weight – he thought the weights may have been wrong In 1865 Newlands suggested the ‘Law of Octaves’. He suggested that elements occurred in pairs that shared similar properties but that their atomic weights differed by 8.

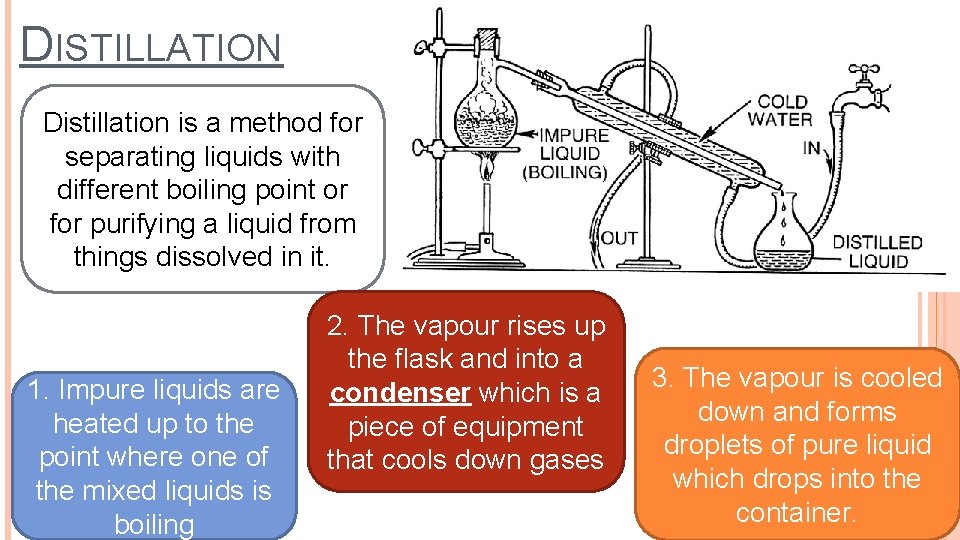
DISTILLATION Distillation is a method for separating liquids with different boiling point or for purifying a liquid from things dissolved in it. 1. Impure liquids are heated up to the point where one of the mixed liquids is boiling 2. The vapour rises up the flask and into a condenser which is a piece of equipment that cools down gases 3. The vapour is cooled down and forms droplets of pure liquid which drops into the container.

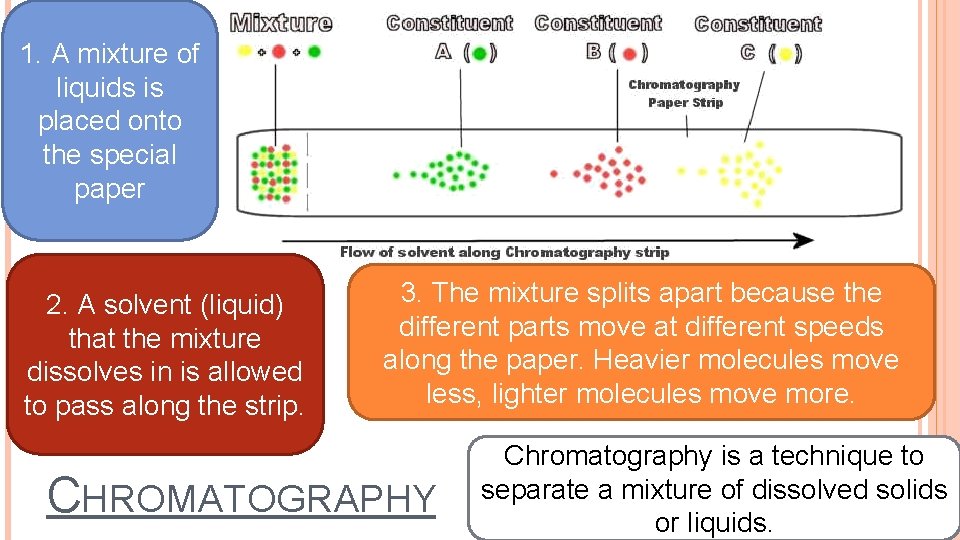
1. A mixture of liquids is placed onto the special paper 2. A solvent (liquid) that the mixture dissolves in is allowed to pass along the strip. 3. The mixture splits apart because the different parts move at different speeds along the paper. Heavier molecules move less, lighter molecules move more. CHROMATOGRAPHY Chromatography is a technique to separate a mixture of dissolved solids or liquids.

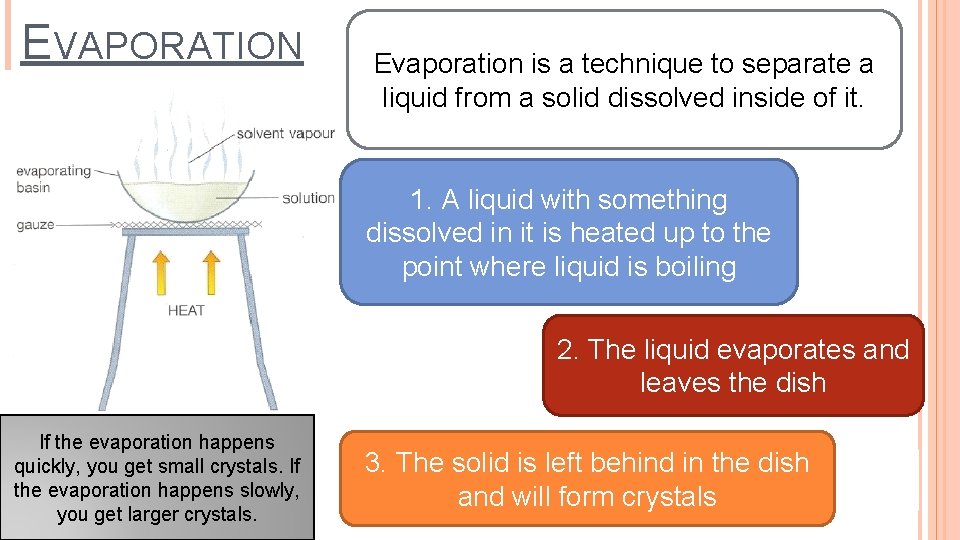
EVAPORATION Evaporation is a technique to separate a liquid from a solid dissolved inside of it. 1. A liquid with something dissolved in it is heated up to the point where liquid is boiling 2. The liquid evaporates and leaves the dish If the evaporation happens quickly, you get small crystals. If the evaporation happens slowly, you get larger crystals. 3. The solid is left behind in the dish and will form crystals

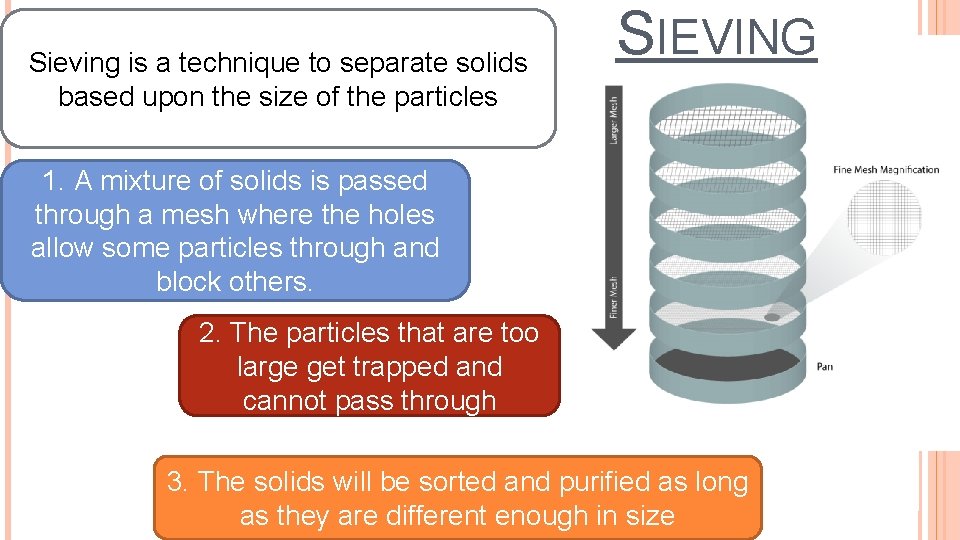
Sieving is a technique to separate solids based upon the size of the particles SIEVING 1. A mixture of solids is passed through a mesh where the holes allow some particles through and block others. 2. The particles that are too large get trapped and cannot pass through 3. The solids will be sorted and purified as long as they are different enough in size

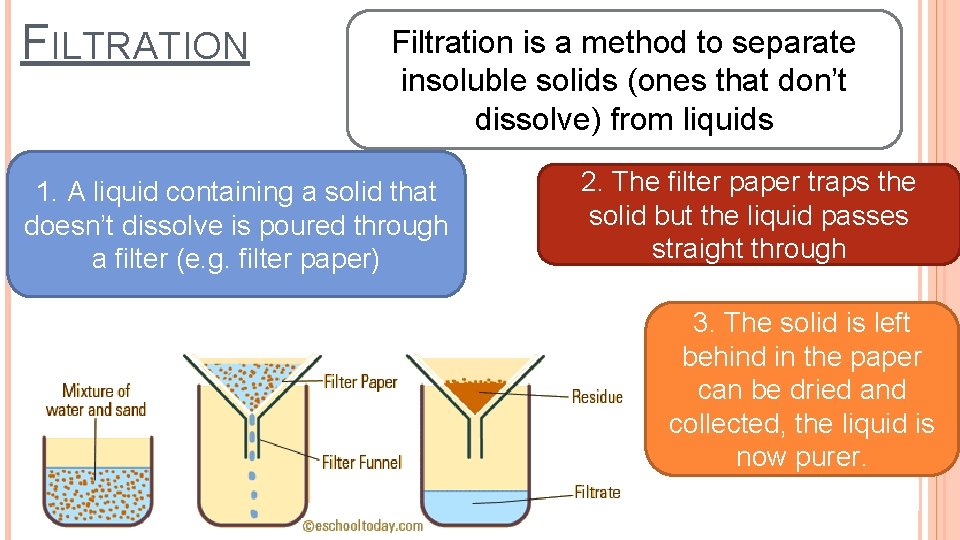
FILTRATION Filtration is a method to separate insoluble solids (ones that don’t dissolve) from liquids 1. A liquid containing a solid that doesn’t dissolve is poured through a filter (e. g. filter paper) 2. The filter paper traps the solid but the liquid passes straight through 3. The solid is left behind in the paper can be dried and collected, the liquid is now purer.

SORTING BY OTHER PROPERTIES There a number of other ways in which we can separate mixtures, based upon what properties they have Magnetic or Not You can separate a mixture of magnetic and non-magnetic materials by using a magnet. Colour You can use the appearance of the different parts of the mixture, for example picking apart different colours that you can see. Natural separation Some substances, for example water and oil, will naturally separate if left alone to settle.