ATOMIC STRUCTURE A guide for GCSE students 2010

















- Slides: 93

ATOMIC STRUCTURE A guide for GCSE students 2010 KNOCKHARDY PUBLISHING SPECIFICATIONS

ATOMIC STRUCTURE INTRODUCTION This Powerpoint show is one of several produced to help students understand selected GCSE Chemistry topics. It is based on the requirements of the AQA specification but is suitable for other examination boards. Individual students may use the material at home for revision purposes and it can also prove useful for classroom teaching with an interactive white board. Accompanying notes on this, and the full range of AS and A 2 Chemistry topics, are available from the KNOCKHARDY SCIENCE WEBSITE at. . . www. knockhardy. org. uk/gcse. htm All diagrams and animations in this Powerpoint are original and created by Jonathan Hopton. Permission must be obtained for their use in any commercial work.

ATOMIC STRUCTURE CONTENTS • Rutherford’s experiment • Structure of atoms • Mass number and atomic number • Atoms and ions • Isotopes • Electronic structures of the first 20 elements

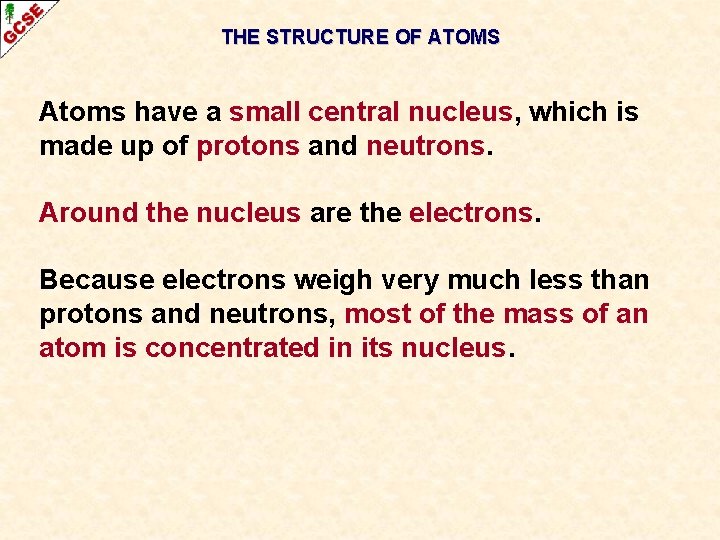
THE STRUCTURE OF ATOMS Atoms have a small central nucleus, which is made up of protons and neutrons. Around the nucleus are the electrons. Because electrons weigh very much less than protons and neutrons, most of the mass of an atom is concentrated in its nucleus.

ATOMIC STRUCTURE RUTHERFORD’S EXPERIMENT

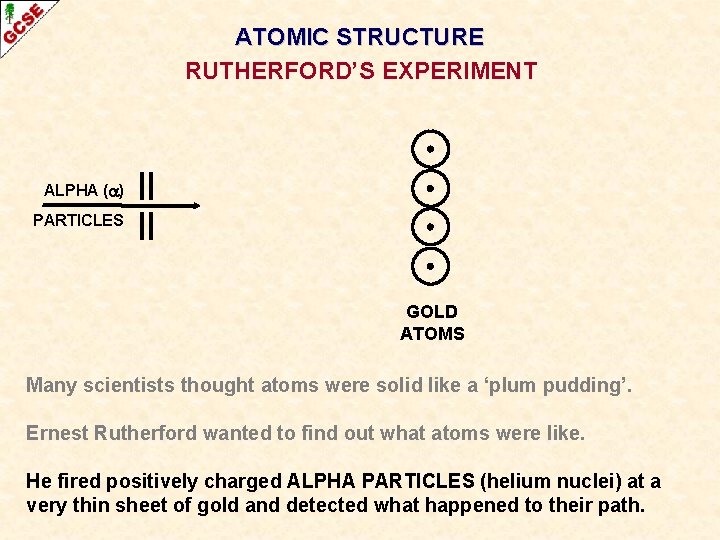
ATOMIC STRUCTURE RUTHERFORD’S EXPERIMENT Many scientists thought atoms were solid like a ‘plum pudding’. Ernest Rutherford wanted to find out what atoms were like.

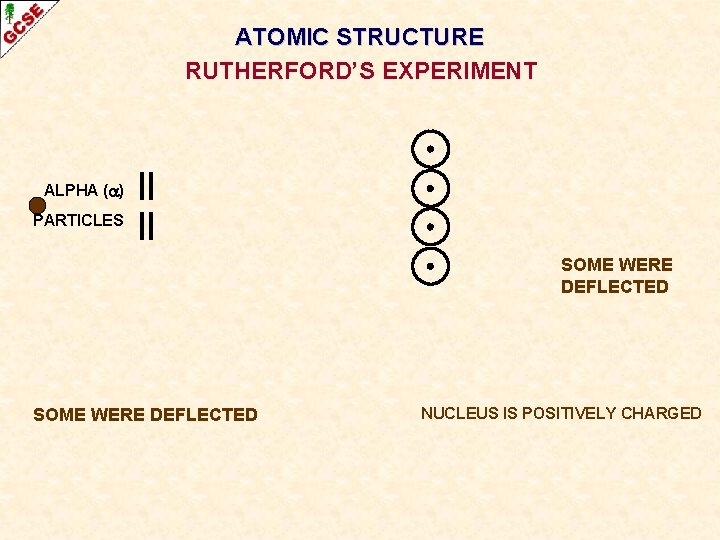
ATOMIC STRUCTURE RUTHERFORD’S EXPERIMENT ALPHA ( ) PARTICLES GOLD ATOMS Many scientists thought atoms were solid like a ‘plum pudding’. Ernest Rutherford wanted to find out what atoms were like. He fired positively charged ALPHA PARTICLES (helium nuclei) at a very thin sheet of gold and detected what happened to their path.

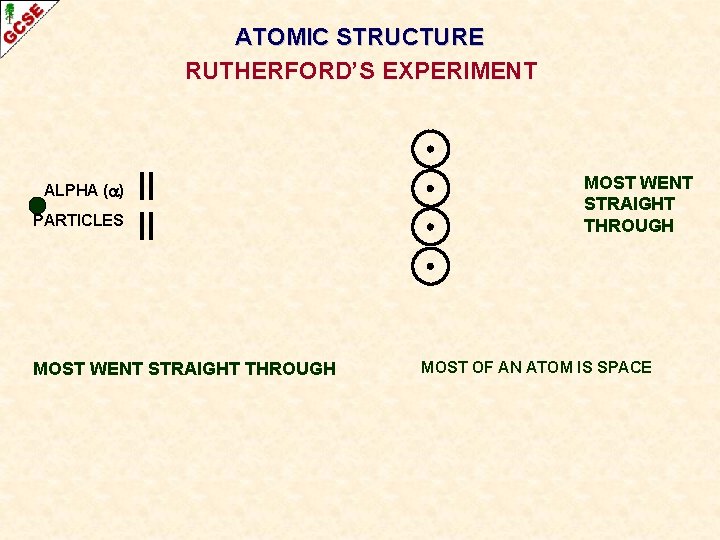
ATOMIC STRUCTURE RUTHERFORD’S EXPERIMENT ALPHA ( ) PARTICLES MOST WENT STRAIGHT THROUGH MOST OF AN ATOM IS SPACE

ATOMIC STRUCTURE RUTHERFORD’S EXPERIMENT ALPHA ( ) PARTICLES SOME WERE DEFLECTED NUCLEUS IS POSITIVELY CHARGED

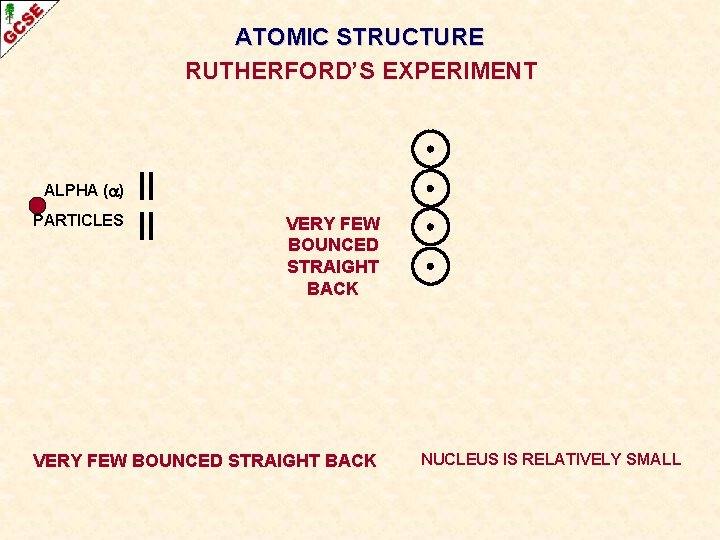
ATOMIC STRUCTURE RUTHERFORD’S EXPERIMENT ALPHA ( ) PARTICLES VERY FEW BOUNCED STRAIGHT BACK NUCLEUS IS RELATIVELY SMALL

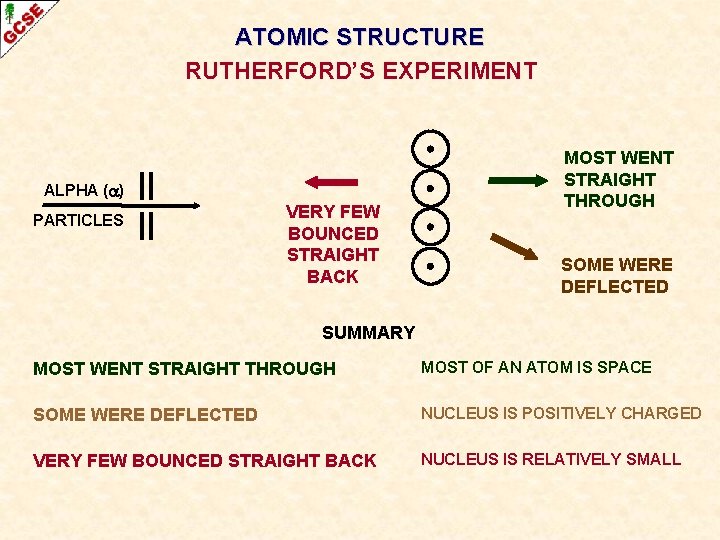
ATOMIC STRUCTURE RUTHERFORD’S EXPERIMENT ALPHA ( ) PARTICLES VERY FEW BOUNCED STRAIGHT BACK MOST WENT STRAIGHT THROUGH SOME WERE DEFLECTED SUMMARY MOST WENT STRAIGHT THROUGH MOST OF AN ATOM IS SPACE SOME WERE DEFLECTED NUCLEUS IS POSITIVELY CHARGED VERY FEW BOUNCED STRAIGHT BACK NUCLEUS IS RELATIVELY SMALL

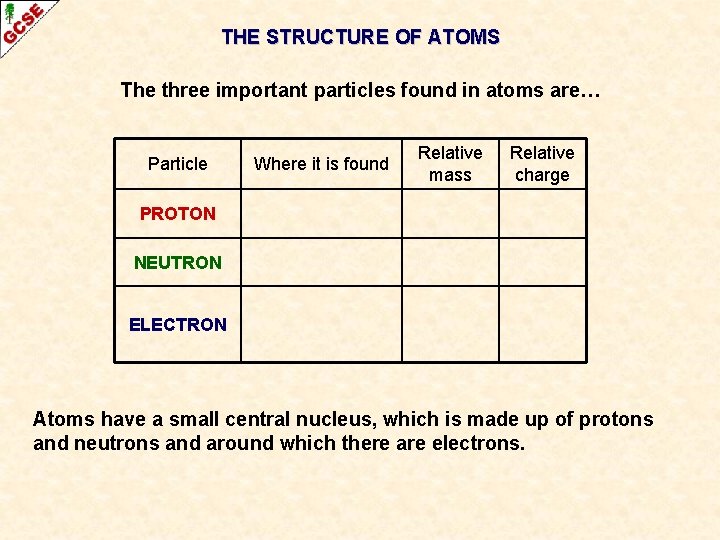
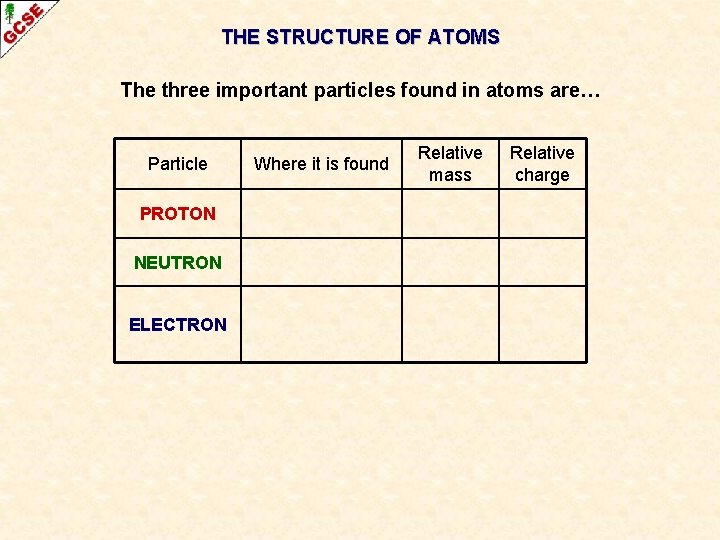
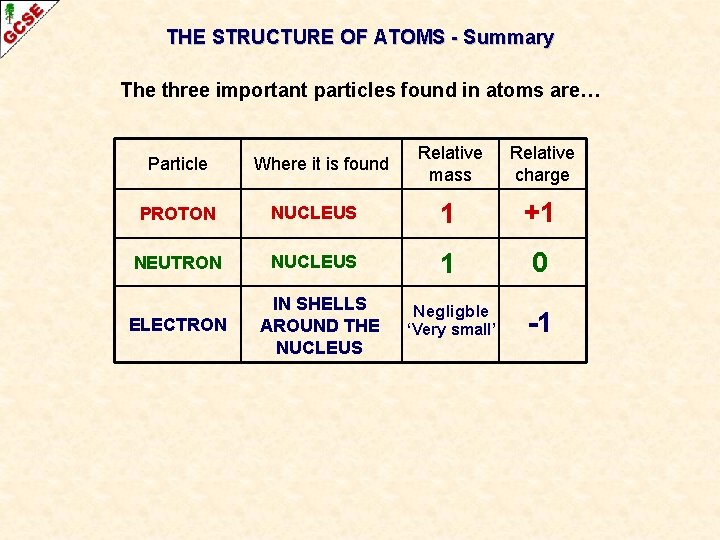
THE STRUCTURE OF ATOMS The three important particles found in atoms are… Particle Where it is found Relative mass Relative charge PROTON NEUTRON ELECTRON Atoms have a small central nucleus, which is made up of protons and neutrons and around which there are electrons.

THE STRUCTURE OF ATOMS The three important particles found in atoms are… Particle PROTON NEUTRON ELECTRON Where it is found Relative mass Relative charge

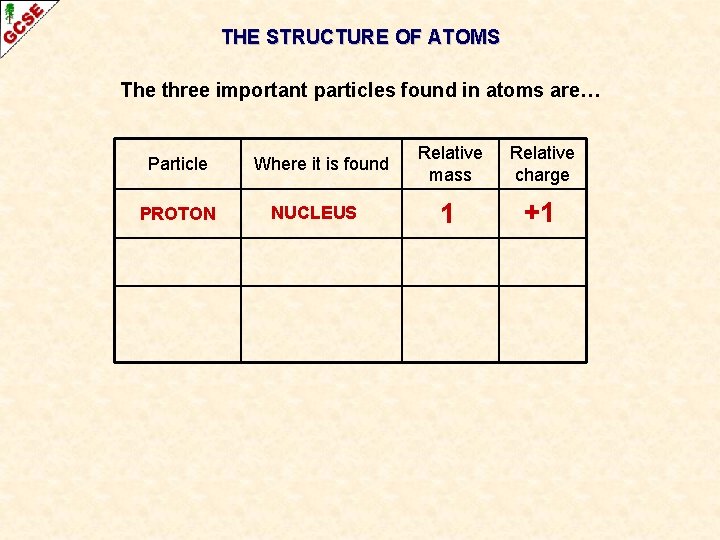
THE STRUCTURE OF ATOMS The three important particles found in atoms are… Particle PROTON Where it is found NUCLEUS Relative mass Relative charge 1 +1

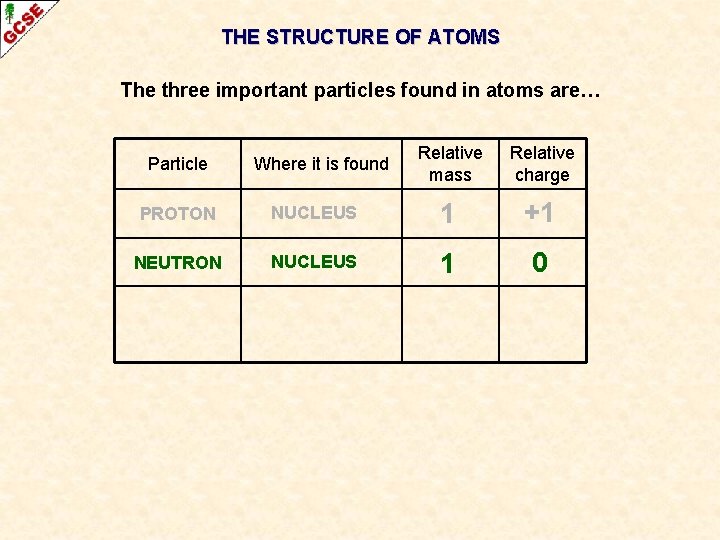
THE STRUCTURE OF ATOMS The three important particles found in atoms are… Particle Where it is found Relative mass Relative charge PROTON NUCLEUS 1 +1 NEUTRON NUCLEUS 1 0

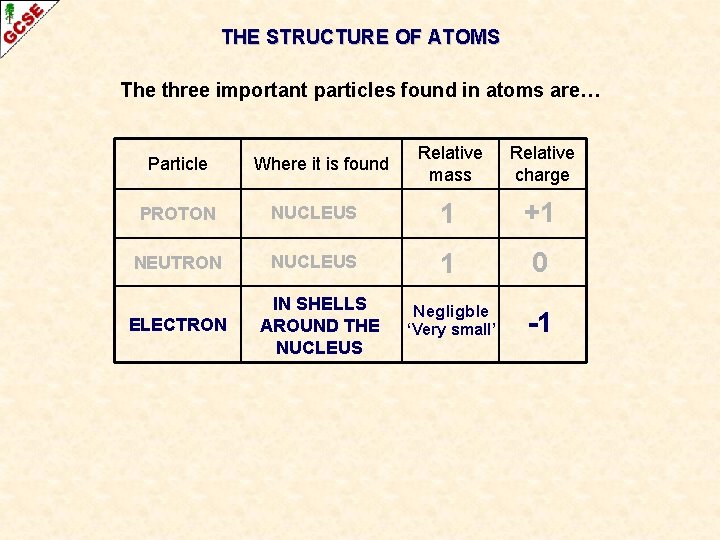
THE STRUCTURE OF ATOMS The three important particles found in atoms are… Particle Where it is found Relative mass Relative charge PROTON NUCLEUS 1 +1 NEUTRON NUCLEUS 1 0 Negligble -1 ELECTRON IN SHELLS AROUND THE NUCLEUS ‘Very small’

THE STRUCTURE OF ATOMS - Summary The three important particles found in atoms are… Particle Where it is found Relative mass Relative charge PROTON NUCLEUS 1 +1 NEUTRON NUCLEUS 1 0 Negligble -1 ELECTRON IN SHELLS AROUND THE NUCLEUS ‘Very small’

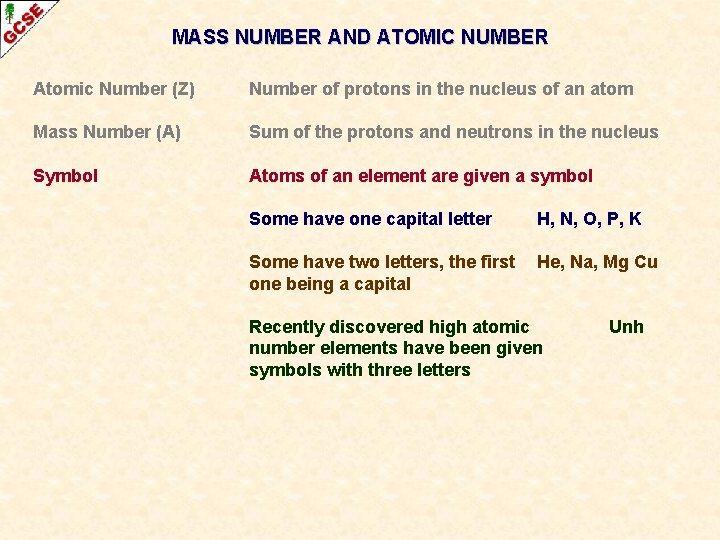
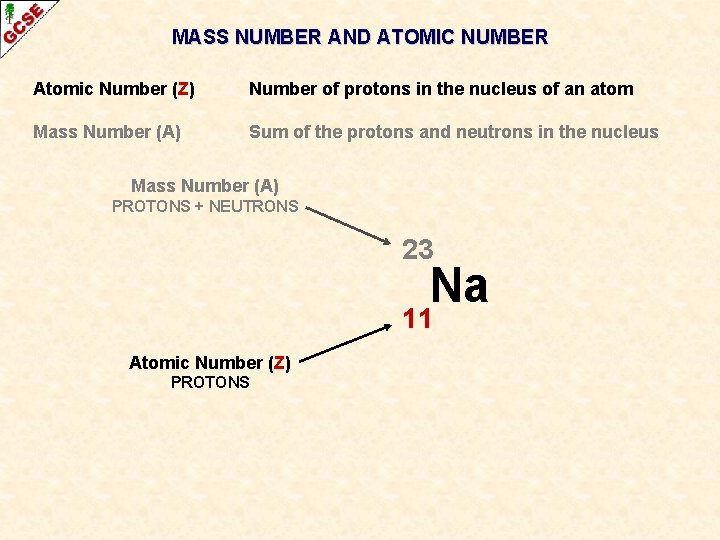
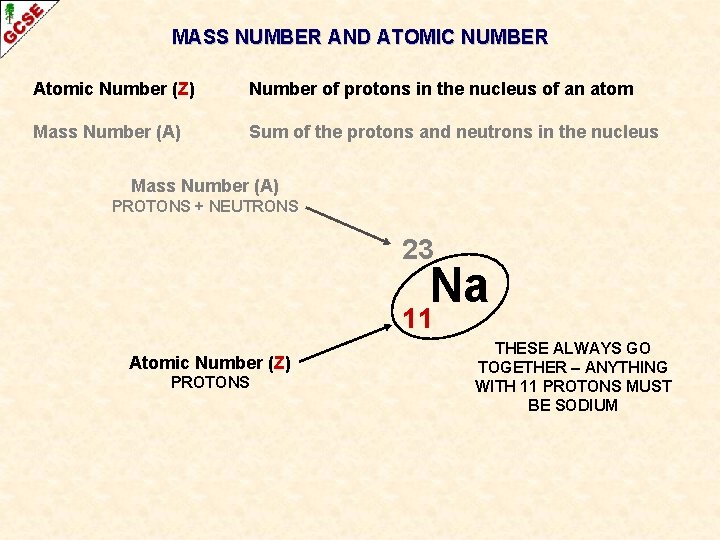
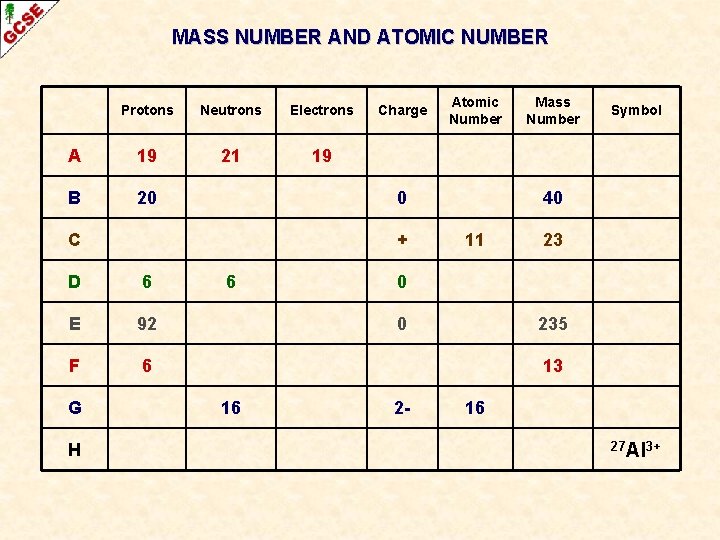
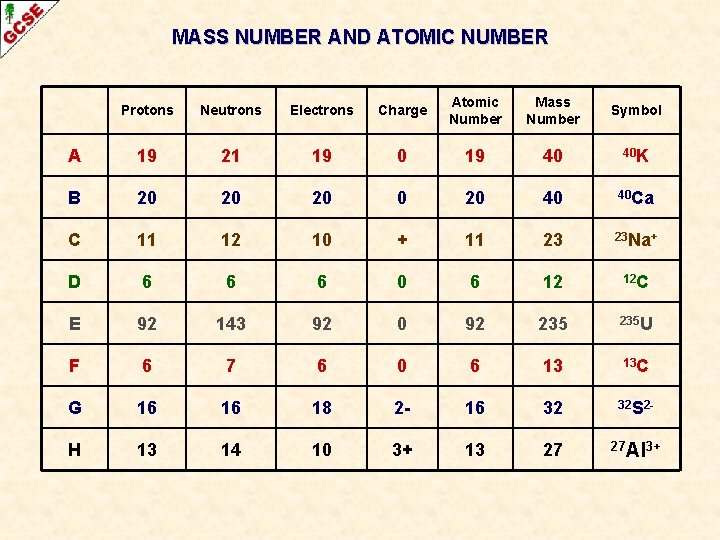
MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an atom Mass Number (A) Sum of the protons and neutrons in the nucleus

MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an atom Mass Number (A) Sum of the protons and neutrons in the nucleus Symbol Atoms of an element are given a symbol Some have one capital letter H, N, O, P, K Some have two letters, the first one being a capital He, Na, Mg Cu Recently discovered high atomic number elements have been given symbols with three letters Unh

MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an atom Mass Number (A) Sum of the protons and neutrons in the nucleus Symbol Atoms of an element are given a symbol Some have one capital letter H, N, O, P, K Some have two letters, the first one being a capital He, Na, Mg Cu Recently discovered high atomic number elements have been given symbols with three letters When necessary, the atomic number and the mass number can be written alongside the symbol Unh

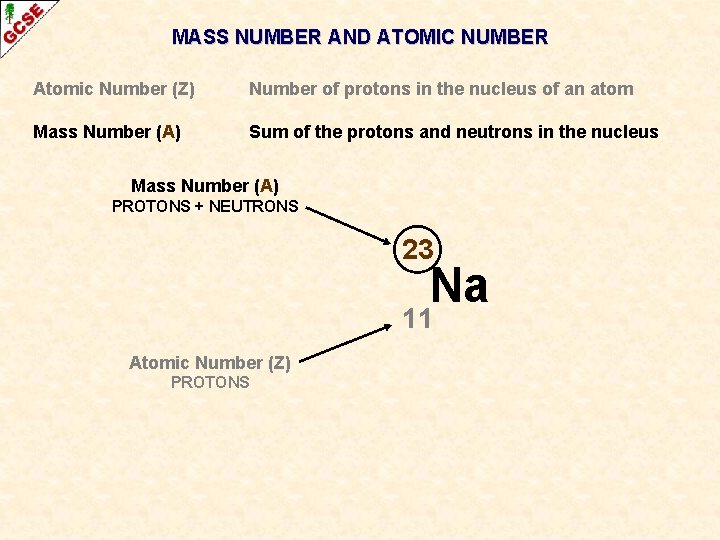
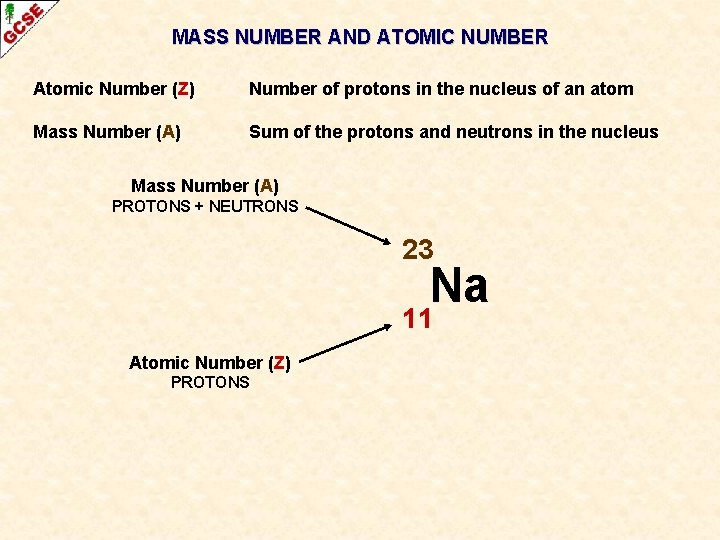
MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an atom Mass Number (A) Sum of the protons and neutrons in the nucleus 23 Na 11 When necessary, the atomic number and the mass number can be written alongside the symbol

MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an atom Mass Number (A) Sum of the protons and neutrons in the nucleus Mass Number (A) PROTONS + NEUTRONS 23 Na 11 Atomic Number (Z) PROTONS

MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an atom Mass Number (A) Sum of the protons and neutrons in the nucleus Mass Number (A) PROTONS + NEUTRONS 23 Na 11 Atomic Number (Z) PROTONS THESE ALWAYS GO TOGETHER – ANYTHING WITH 11 PROTONS MUST BE SODIUM

MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an atom Mass Number (A) Sum of the protons and neutrons in the nucleus Mass Number (A) PROTONS + NEUTRONS 23 Na 11 Atomic Number (Z) PROTONS

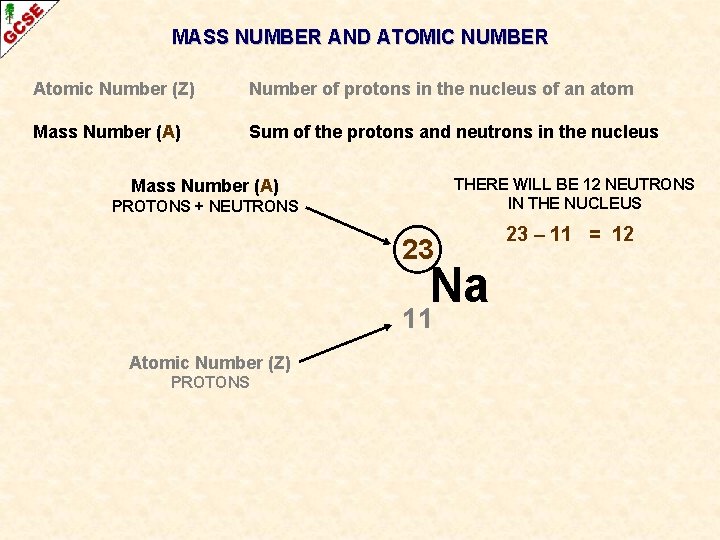
MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an atom Mass Number (A) Sum of the protons and neutrons in the nucleus THERE WILL BE 12 NEUTRONS IN THE NUCLEUS Mass Number (A) PROTONS + NEUTRONS 23 Na 11 Atomic Number (Z) PROTONS 23 – 11 = 12

MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an atom Mass Number (A) Sum of the protons and neutrons in the nucleus Mass Number (A) PROTONS + NEUTRONS 23 Na 11 Atomic Number (Z) PROTONS

ATOMS AND IONS

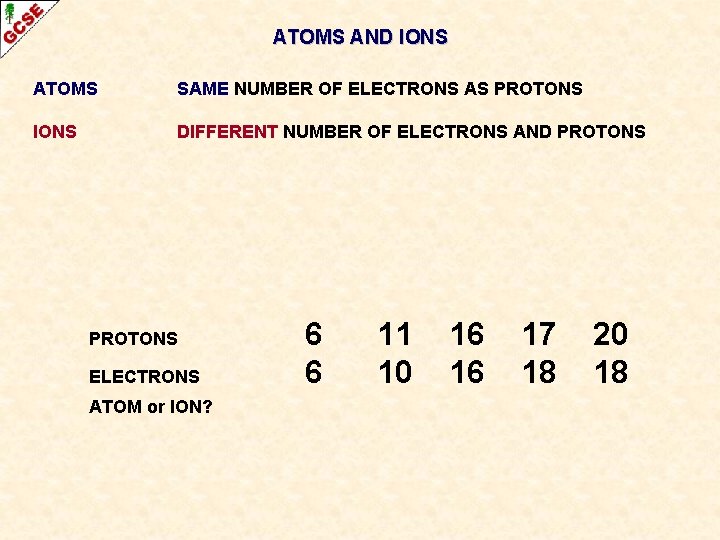
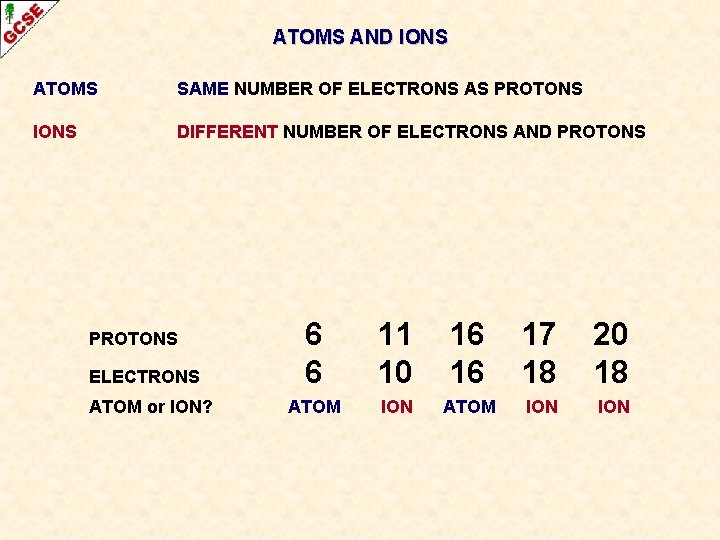
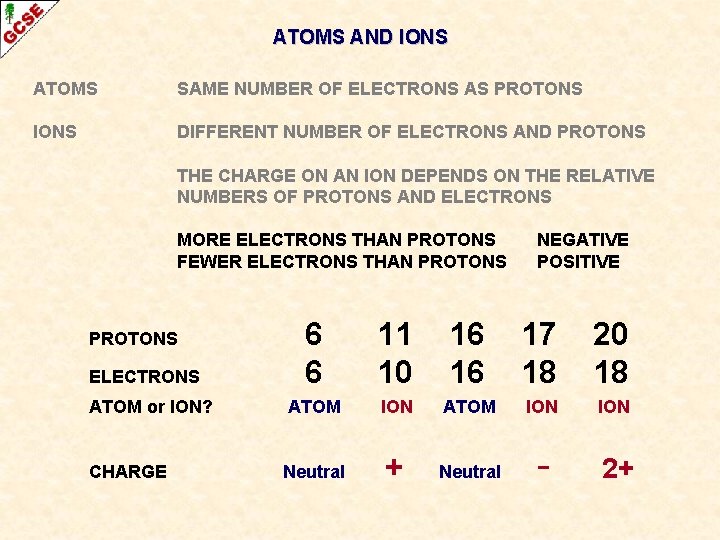
ATOMS AND IONS ATOMS SAME NUMBER OF ELECTRONS AS PROTONS IONS DIFFERENT NUMBER OF ELECTRONS AND PROTONS

ATOMS AND IONS ATOMS SAME NUMBER OF ELECTRONS AS PROTONS IONS DIFFERENT NUMBER OF ELECTRONS AND PROTONS ELECTRONS ATOM or ION? 6 6 11 10 16 16 17 18 20 18

ATOMS AND IONS ATOMS SAME NUMBER OF ELECTRONS AS PROTONS IONS DIFFERENT NUMBER OF ELECTRONS AND PROTONS ELECTRONS ATOM or ION? 6 6 11 10 16 16 17 18 20 18 ATOM ION ION

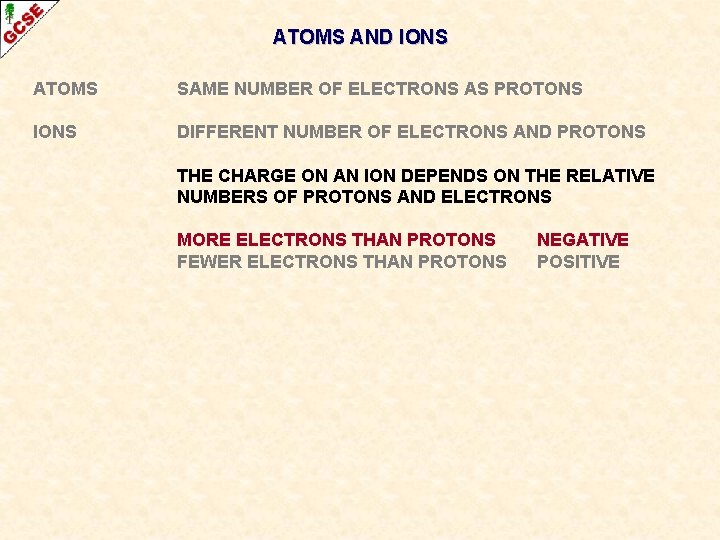
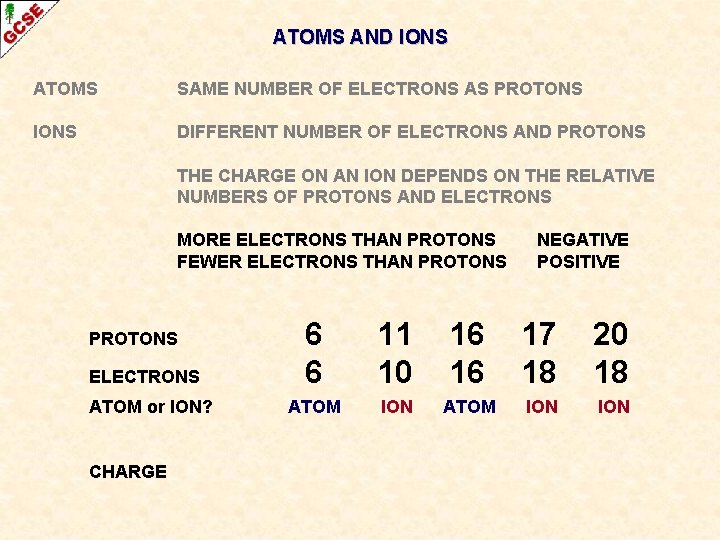
ATOMS AND IONS ATOMS SAME NUMBER OF ELECTRONS AS PROTONS IONS DIFFERENT NUMBER OF ELECTRONS AND PROTONS THE CHARGE ON AN ION DEPENDS ON THE RELATIVE NUMBERS OF PROTONS AND ELECTRONS

ATOMS AND IONS ATOMS SAME NUMBER OF ELECTRONS AS PROTONS IONS DIFFERENT NUMBER OF ELECTRONS AND PROTONS THE CHARGE ON AN ION DEPENDS ON THE RELATIVE NUMBERS OF PROTONS AND ELECTRONS MORE ELECTRONS THAN PROTONS FEWER ELECTRONS THAN PROTONS NEGATIVE POSITIVE

ATOMS AND IONS ATOMS SAME NUMBER OF ELECTRONS AS PROTONS IONS DIFFERENT NUMBER OF ELECTRONS AND PROTONS THE CHARGE ON AN ION DEPENDS ON THE RELATIVE NUMBERS OF PROTONS AND ELECTRONS MORE ELECTRONS THAN PROTONS FEWER ELECTRONS THAN PROTONS NEGATIVE POSITIVE

ATOMS AND IONS ATOMS SAME NUMBER OF ELECTRONS AS PROTONS IONS DIFFERENT NUMBER OF ELECTRONS AND PROTONS THE CHARGE ON AN ION DEPENDS ON THE RELATIVE NUMBERS OF PROTONS AND ELECTRONS MORE ELECTRONS THAN PROTONS FEWER ELECTRONS THAN PROTONS ELECTRONS ATOM or ION? CHARGE NEGATIVE POSITIVE 6 6 11 10 16 16 17 18 20 18 ATOM ION ION

ATOMS AND IONS ATOMS SAME NUMBER OF ELECTRONS AS PROTONS IONS DIFFERENT NUMBER OF ELECTRONS AND PROTONS THE CHARGE ON AN ION DEPENDS ON THE RELATIVE NUMBERS OF PROTONS AND ELECTRONS MORE ELECTRONS THAN PROTONS FEWER ELECTRONS THAN PROTONS NEGATIVE POSITIVE 6 6 11 10 16 16 17 18 20 18 ATOM or ION? ATOM ION ION CHARGE Neutral + Neutral - 2+ PROTONS ELECTRONS

MASS NUMBER AND ATOMIC NUMBER Protons Neutrons Electrons A 19 21 19 B 20 + D 6 E 92 F 6 H Atomic Number 0 C G Charge 6 Mass Number Symbol 40 11 23 0 0 235 13 16 2 - 16 27 Al 3+

MASS NUMBER AND ATOMIC NUMBER Protons Neutrons Electrons Charge Atomic Number Mass Number Symbol A 19 21 19 0 19 40 40 K B 20 20 20 40 40 Ca C 11 12 10 + 11 23 23 Na+ D 6 6 6 0 6 12 12 C E 92 143 92 0 92 235 U F 6 7 6 0 6 13 13 C G 16 16 18 2 - 16 32 32 S 2 - H 13 14 10 3+ 13 27 27 Al 3+

ISOTOPES

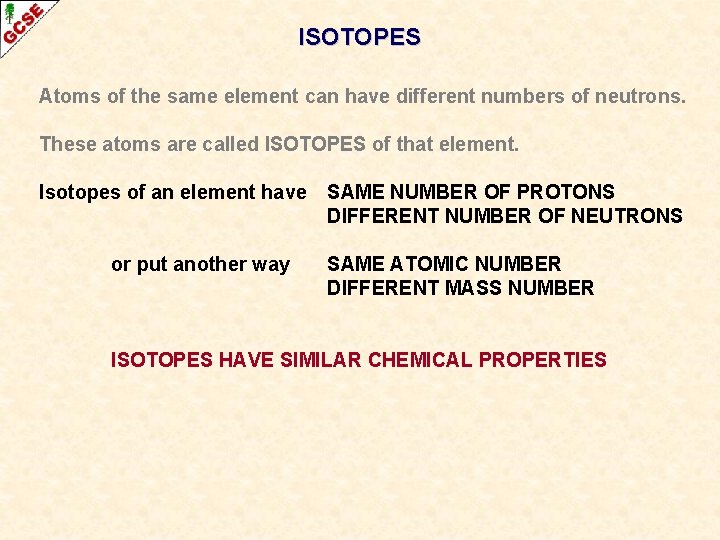
ISOTOPES Atoms of the same element can have different numbers of neutrons. These atoms are called ISOTOPES of that element.

ISOTOPES Atoms of the same element can have different numbers of neutrons. These atoms are called ISOTOPES of that element. Isotopes of an element have SAME NUMBER OF PROTONS DIFFERENT NUMBER OF NEUTRONS

ISOTOPES Atoms of the same element can have different numbers of neutrons. These atoms are called ISOTOPES of that element. Isotopes of an element have or put another way SAME NUMBER OF PROTONS DIFFERENT NUMBER OF NEUTRONS SAME ATOMIC NUMBER DIFFERENT MASS NUMBER

ISOTOPES Atoms of the same element can have different numbers of neutrons. These atoms are called ISOTOPES of that element. Isotopes of an element have or put another way SAME NUMBER OF PROTONS DIFFERENT NUMBER OF NEUTRONS SAME ATOMIC NUMBER DIFFERENT MASS NUMBER ISOTOPES HAVE SIMILAR CHEMICAL PROPERTIES

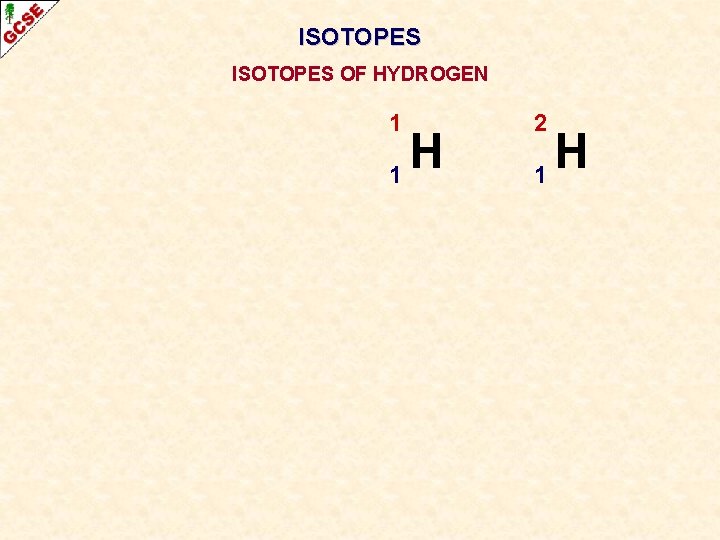
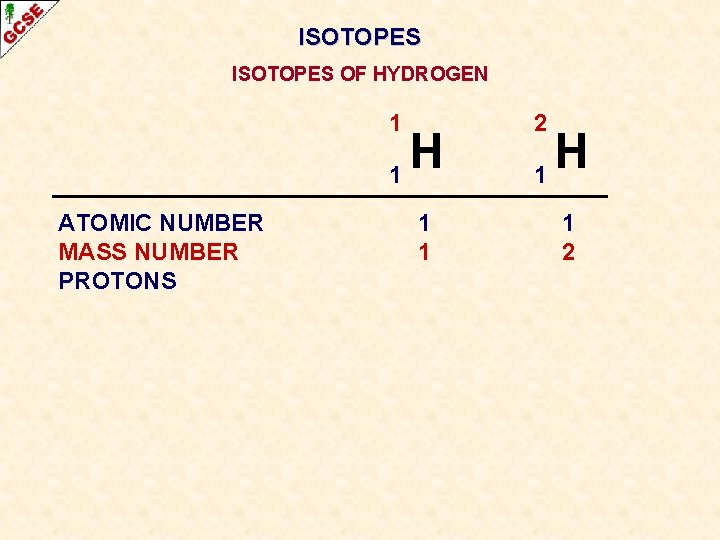
ISOTOPES OF HYDROGEN 1 H 1 2 H 1

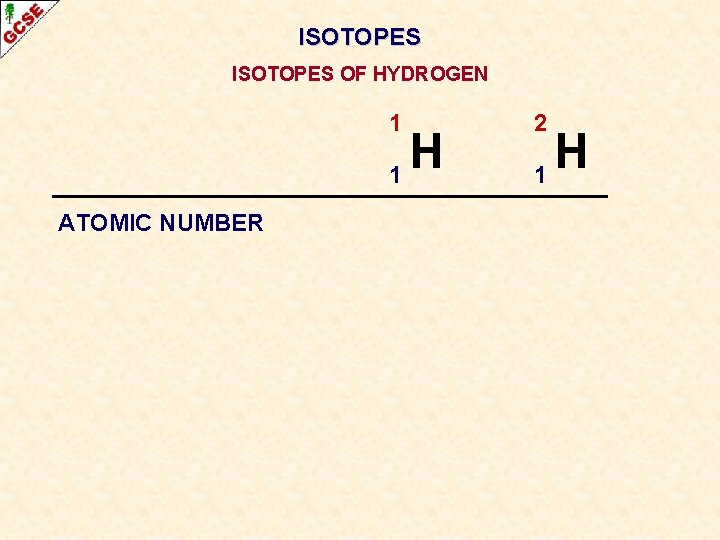
ISOTOPES OF HYDROGEN 1 H 1 ATOMIC NUMBER 2 H 1

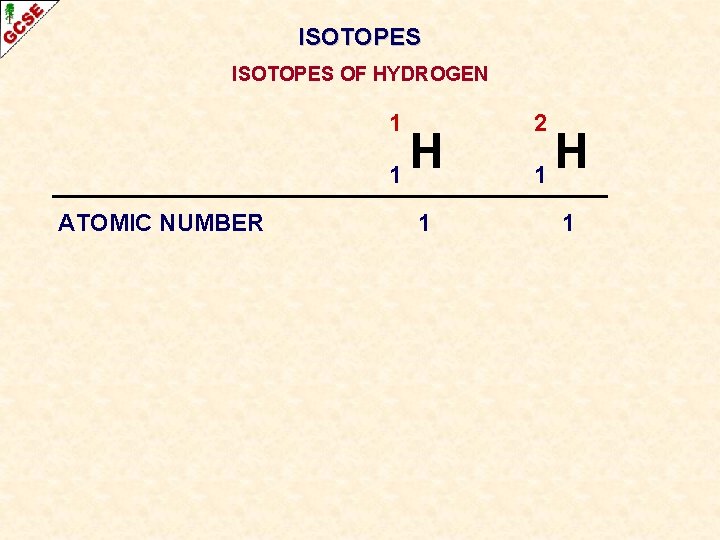
ISOTOPES OF HYDROGEN 1 H 1 ATOMIC NUMBER 1 2 H 1 1

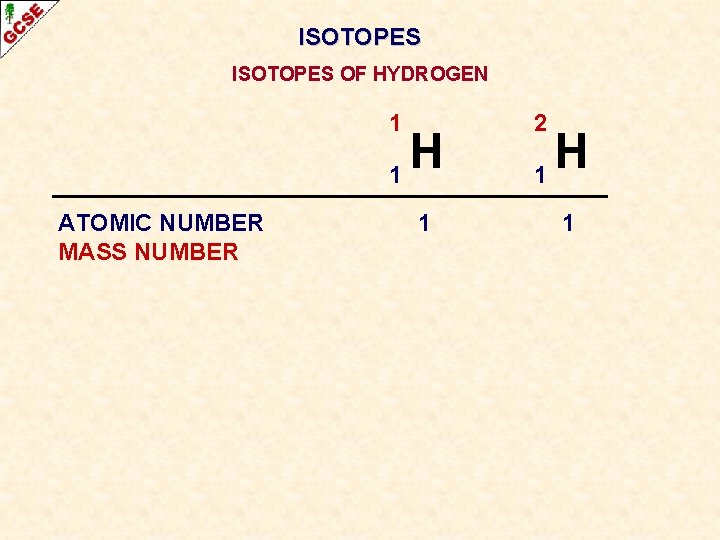
ISOTOPES OF HYDROGEN 1 H 1 ATOMIC NUMBER MASS NUMBER 1 2 H 1 1

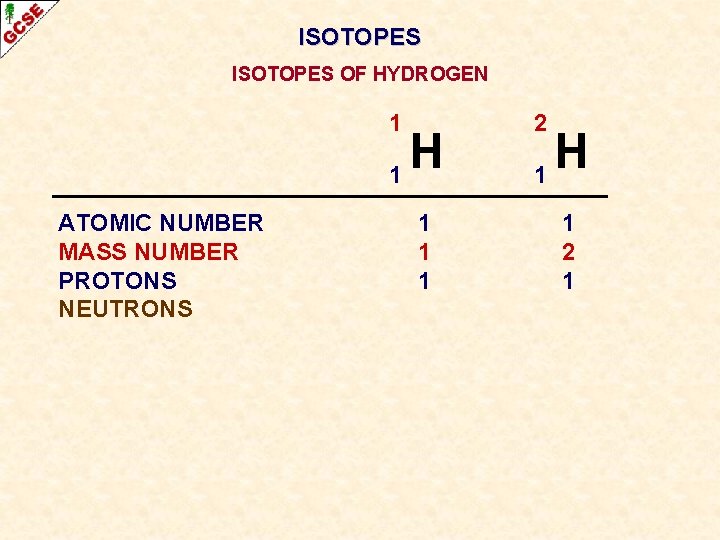
ISOTOPES OF HYDROGEN 1 H 1 ATOMIC NUMBER MASS NUMBER PROTONS 1 1 2 H 1 1 2

ISOTOPES OF HYDROGEN 1 H 1 ATOMIC NUMBER MASS NUMBER PROTONS NEUTRONS 1 1 1 2 H 1 1 2 1

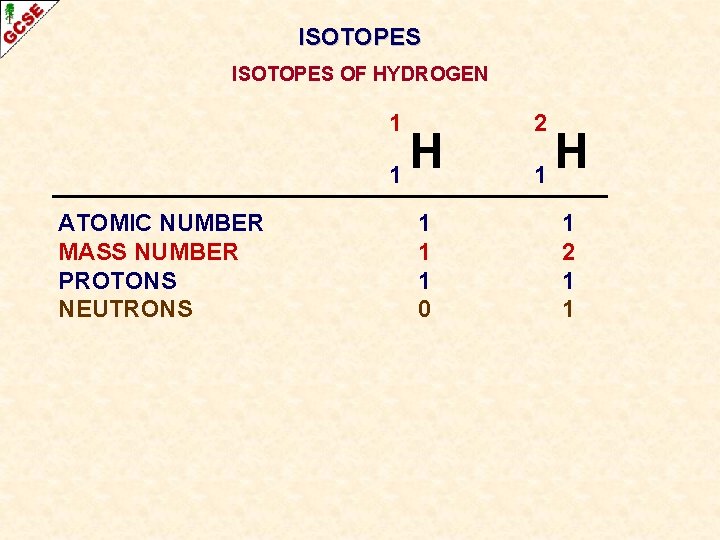
ISOTOPES OF HYDROGEN 1 H 1 ATOMIC NUMBER MASS NUMBER PROTONS NEUTRONS 1 1 1 0 2 H 1 1 2 1 1

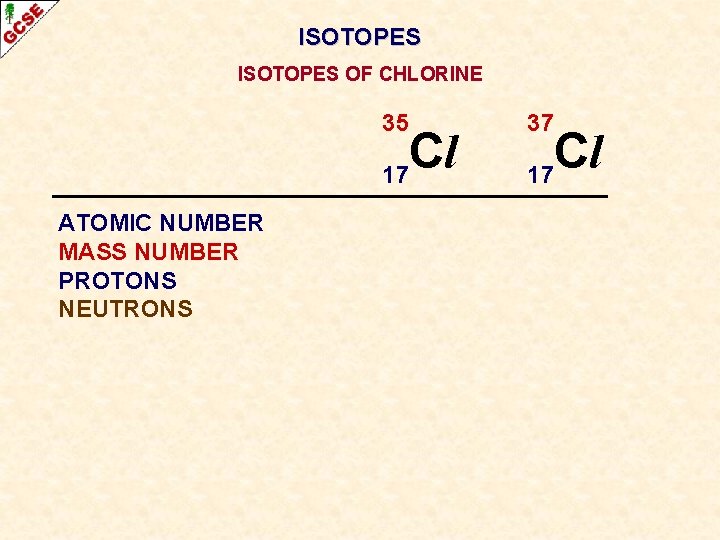
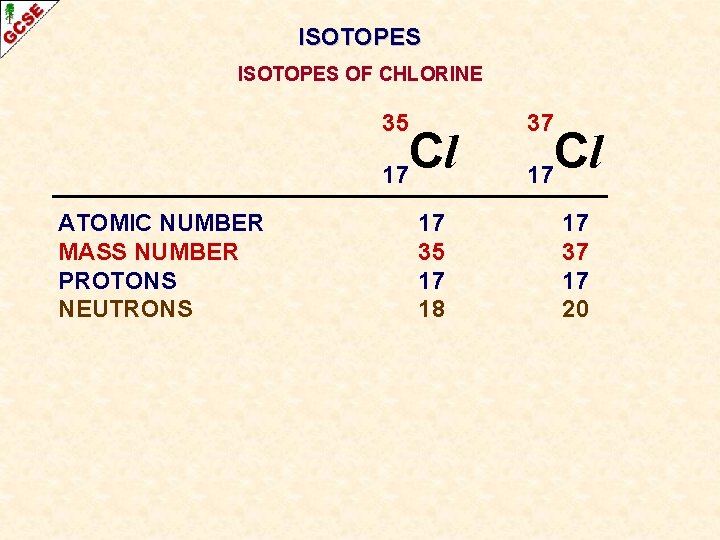
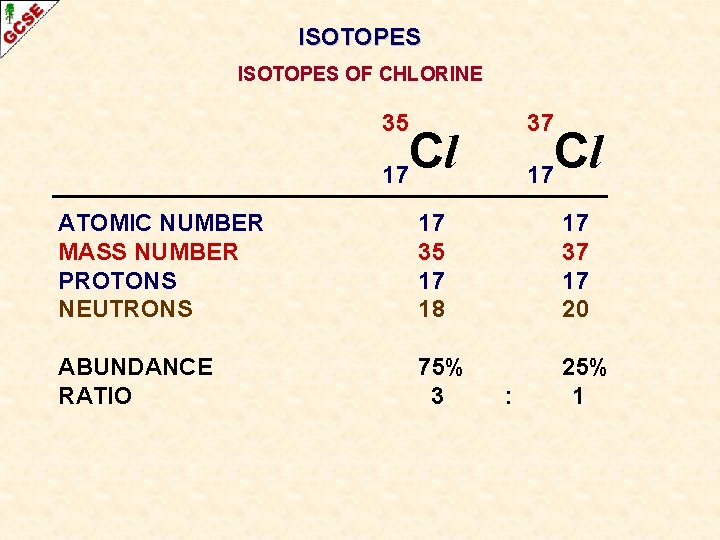
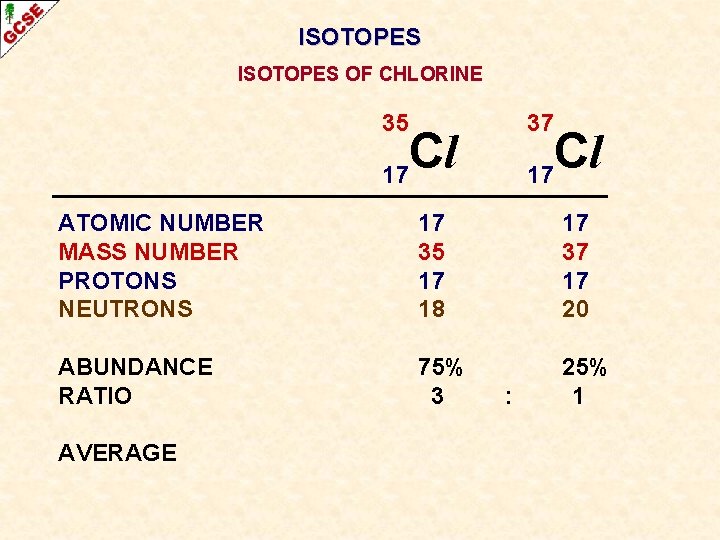
ISOTOPES OF CHLORINE 35 Cl 17 ATOMIC NUMBER MASS NUMBER PROTONS NEUTRONS 37 Cl 17

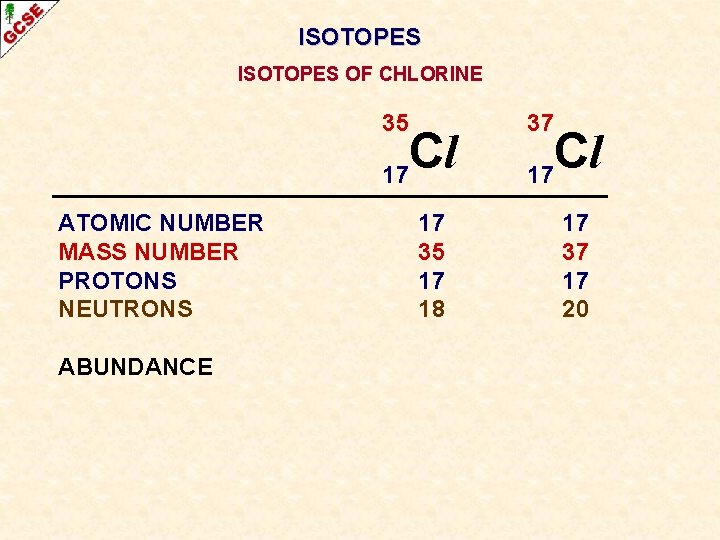
ISOTOPES OF CHLORINE 35 Cl 17 ATOMIC NUMBER MASS NUMBER PROTONS NEUTRONS 17 35 17 18 37 Cl 17 17 37 17 20

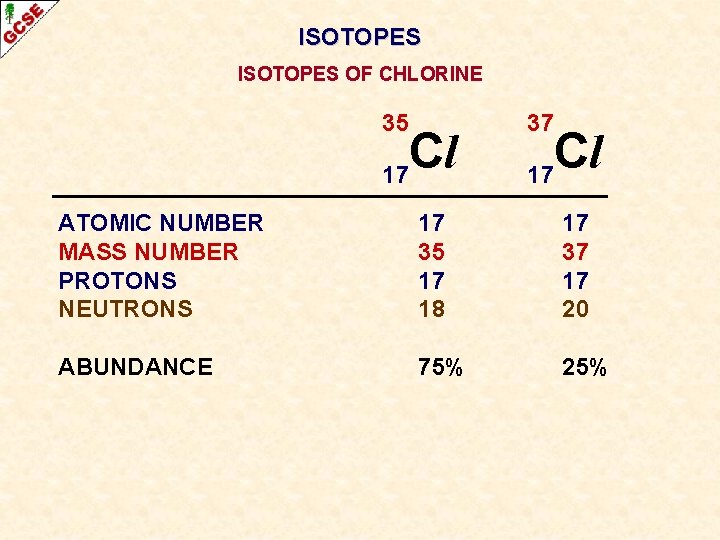
ISOTOPES OF CHLORINE 35 Cl 17 ATOMIC NUMBER MASS NUMBER PROTONS NEUTRONS ABUNDANCE 17 35 17 18 37 Cl 17 17 37 17 20

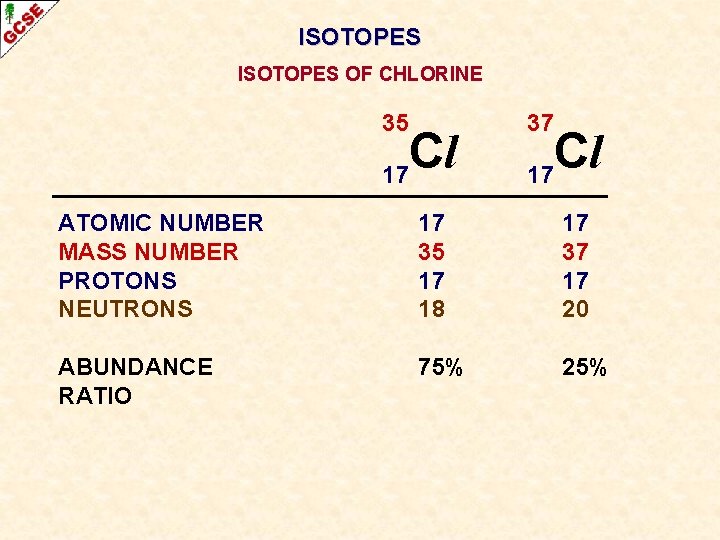
ISOTOPES OF CHLORINE 35 Cl 17 37 Cl 17 ATOMIC NUMBER MASS NUMBER PROTONS NEUTRONS 17 35 17 18 17 37 17 20 ABUNDANCE 75% 25%

ISOTOPES OF CHLORINE 35 Cl 17 37 Cl 17 ATOMIC NUMBER MASS NUMBER PROTONS NEUTRONS 17 35 17 18 17 37 17 20 ABUNDANCE RATIO 75% 25%

ISOTOPES OF CHLORINE 35 37 Cl 17 ATOMIC NUMBER MASS NUMBER PROTONS NEUTRONS 17 35 17 18 17 37 17 20 ABUNDANCE RATIO 75% 3 25% 1 :

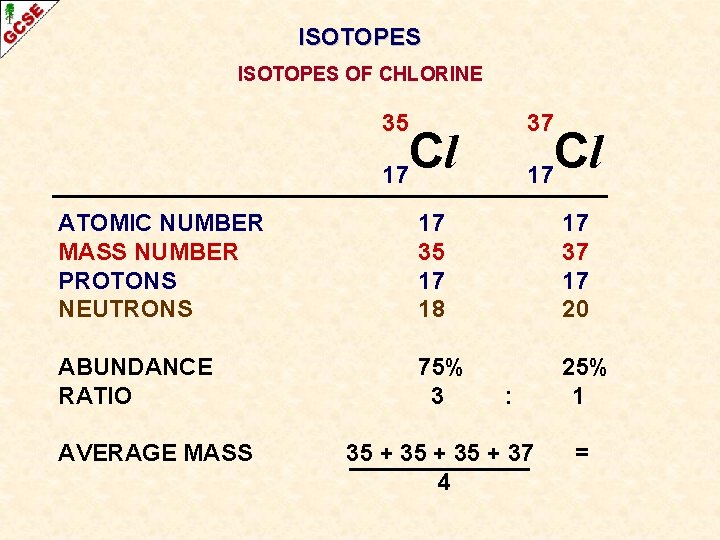
ISOTOPES OF CHLORINE 35 37 Cl 17 ATOMIC NUMBER MASS NUMBER PROTONS NEUTRONS 17 35 17 18 17 37 17 20 ABUNDANCE RATIO 75% 3 25% 1 AVERAGE :

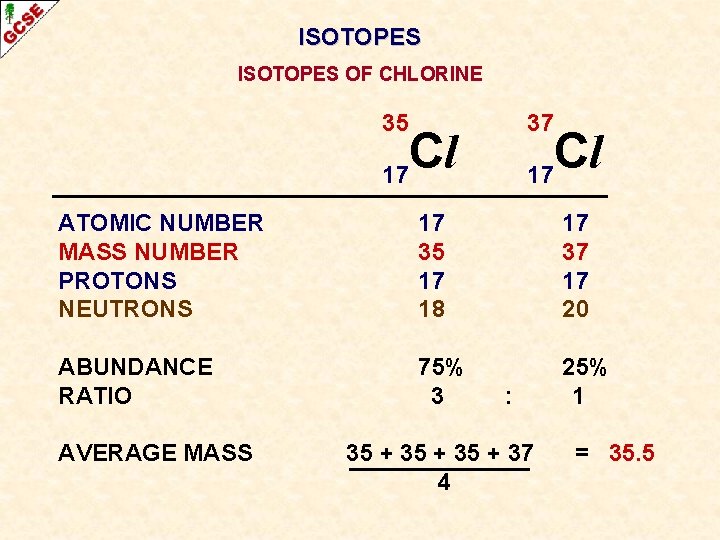
ISOTOPES OF CHLORINE 35 37 Cl 17 ATOMIC NUMBER MASS NUMBER PROTONS NEUTRONS 17 35 17 18 17 37 17 20 ABUNDANCE RATIO 75% 3 25% 1 AVERAGE MASS : 35 + 37 4 =

ISOTOPES OF CHLORINE 35 37 Cl 17 ATOMIC NUMBER MASS NUMBER PROTONS NEUTRONS 17 35 17 18 17 37 17 20 ABUNDANCE RATIO 75% 3 25% 1 AVERAGE MASS : 35 + 37 4 = 35. 5

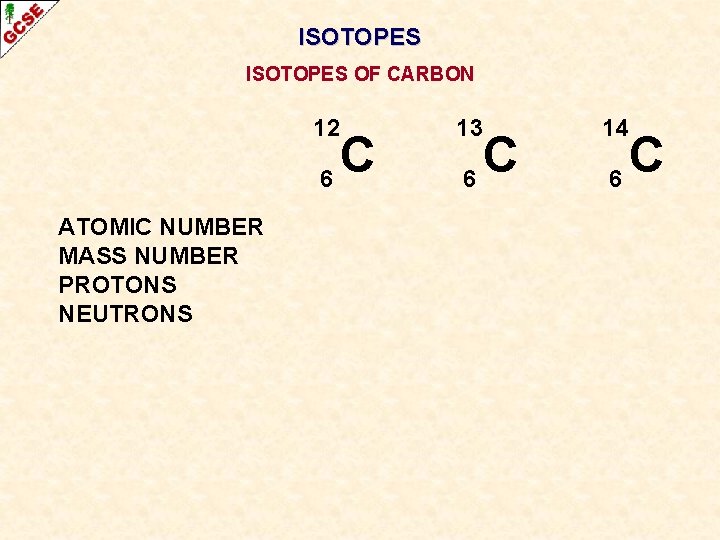
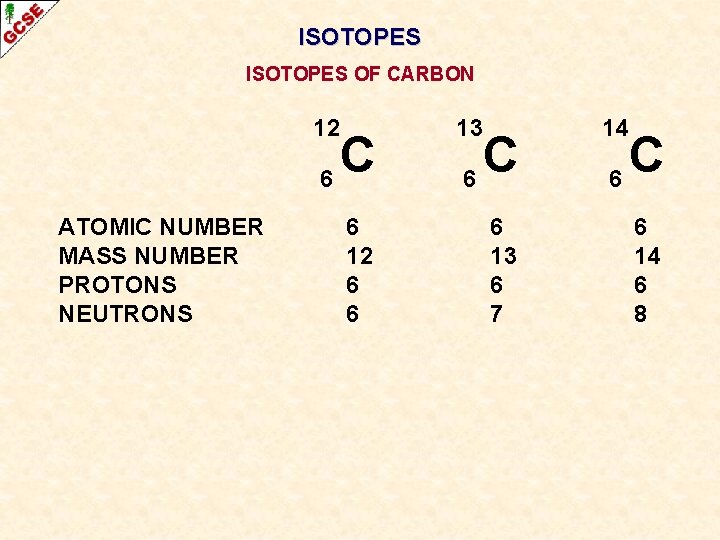
ISOTOPES OF CARBON ATOMIC NUMBER MASS NUMBER PROTONS NEUTRONS 12 13 14 6 6 6 C 6 12 6 6 C 6 13 6 7 C 6 14 6 8

ISOTOPES OF CARBON ATOMIC NUMBER MASS NUMBER PROTONS NEUTRONS 12 13 14 6 6 6 C 6 12 6 6 C 6 13 6 7 C 6 14 6 8

ATOMIC STRUCTURE THE ARRANGEMENT OF ELECTRONS IN ATOMS OF THE FIRST TWENTY ELEMENTS IN THE PERIODIC TABLE

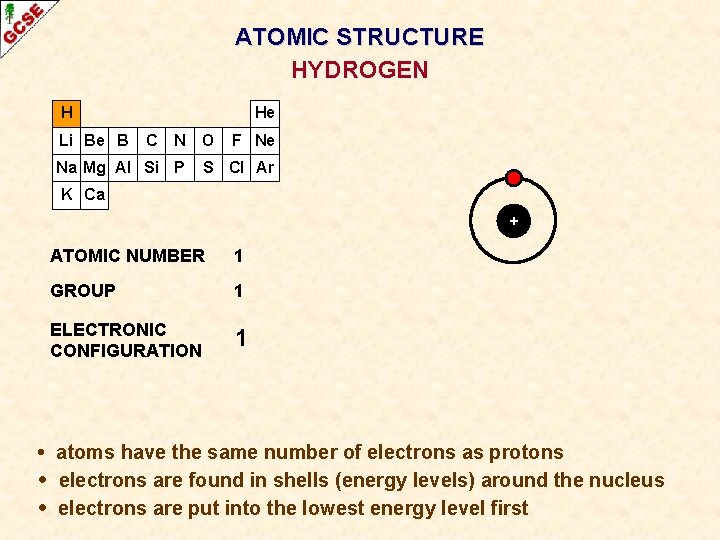
ATOMIC STRUCTURE HYDROGEN H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca + ATOMIC NUMBER 1 GROUP 1 ELECTRONIC CONFIGURATION 1 • atoms have the same number of electrons as protons • electrons are found in shells (energy levels) around the nucleus • electrons are put into the lowest energy level first

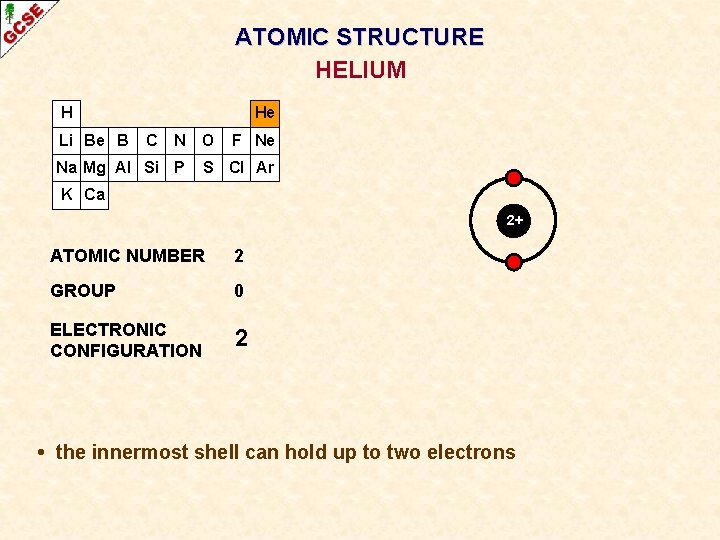
ATOMIC STRUCTURE HELIUM H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 2+ ATOMIC NUMBER 2 GROUP 0 ELECTRONIC CONFIGURATION 2 • the innermost shell can hold up to two electrons

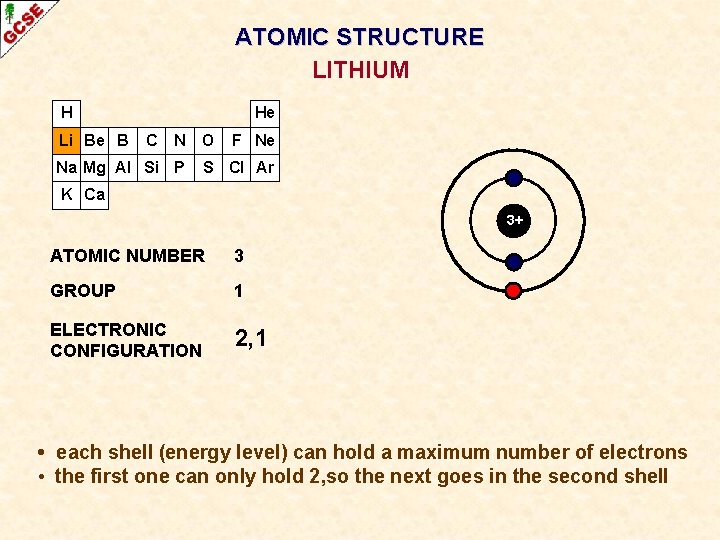
ATOMIC STRUCTURE LITHIUM H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 3+ ATOMIC NUMBER 3 GROUP 1 ELECTRONIC CONFIGURATION 2, 1 • each shell (energy level) can hold a maximum number of electrons • the first one can only hold 2, so the next goes in the second shell

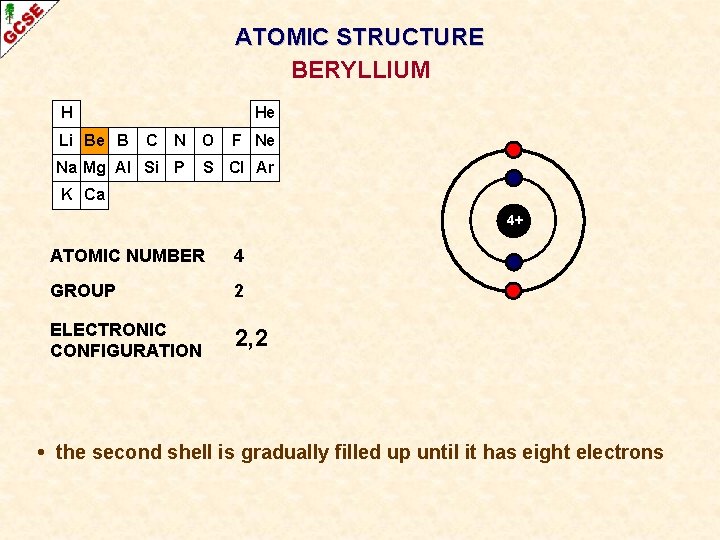
ATOMIC STRUCTURE BERYLLIUM H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 4+ ATOMIC NUMBER 4 GROUP 2 ELECTRONIC CONFIGURATION 2, 2 • the second shell is gradually filled up until it has eight electrons

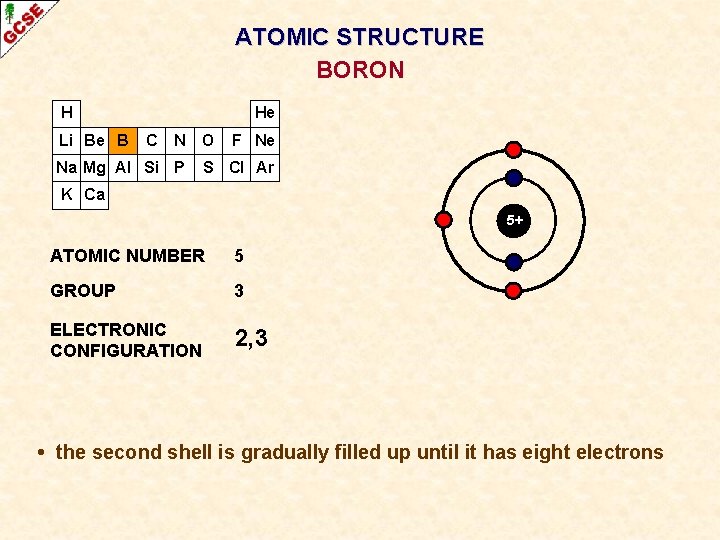
ATOMIC STRUCTURE BORON H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 5+ ATOMIC NUMBER 5 GROUP 3 ELECTRONIC CONFIGURATION 2, 3 • the second shell is gradually filled up until it has eight electrons

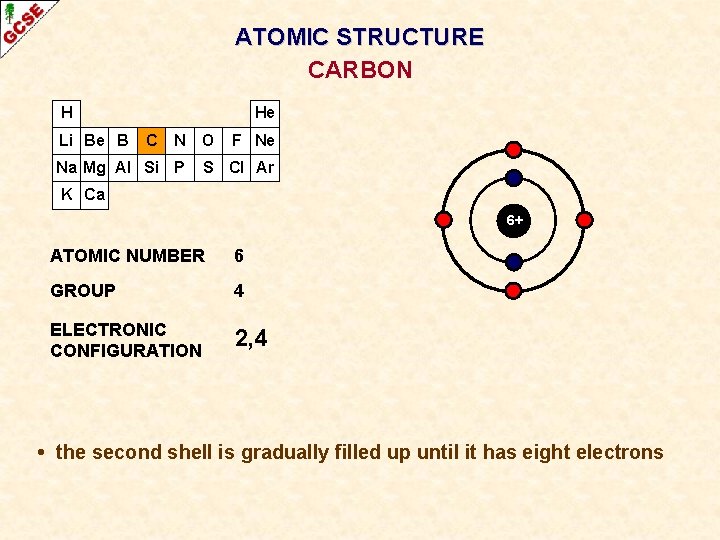
ATOMIC STRUCTURE CARBON H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 6+ ATOMIC NUMBER 6 GROUP 4 ELECTRONIC CONFIGURATION 2, 4 • the second shell is gradually filled up until it has eight electrons

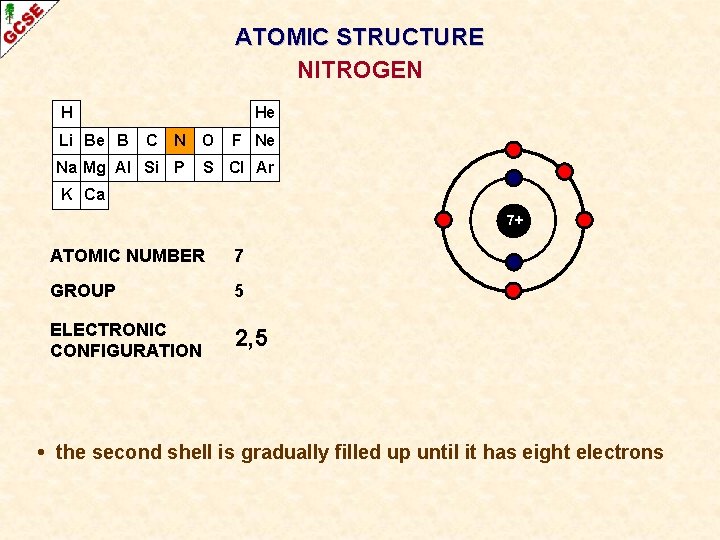
ATOMIC STRUCTURE NITROGEN H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 7+ ATOMIC NUMBER 7 GROUP 5 ELECTRONIC CONFIGURATION 2, 5 • the second shell is gradually filled up until it has eight electrons

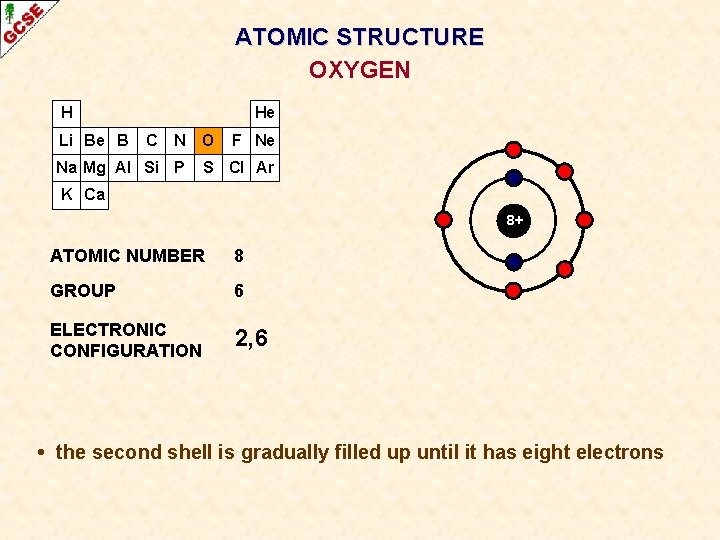
ATOMIC STRUCTURE OXYGEN H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 8+ ATOMIC NUMBER 8 GROUP 6 ELECTRONIC CONFIGURATION 2, 6 • the second shell is gradually filled up until it has eight electrons

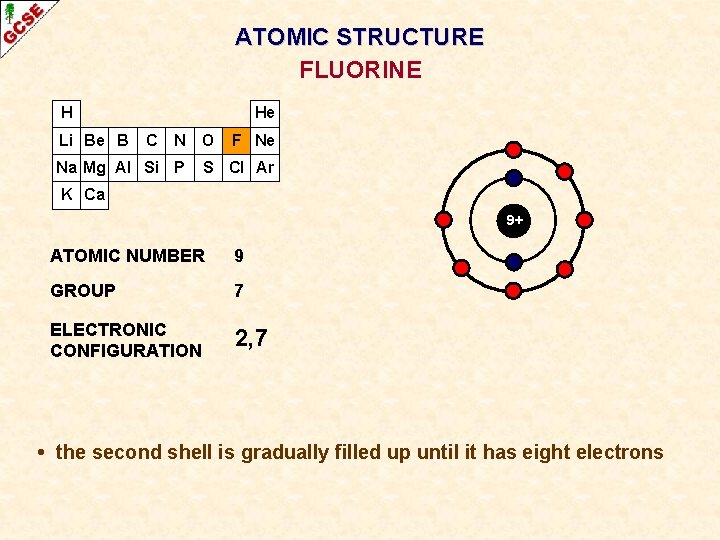
ATOMIC STRUCTURE FLUORINE H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 9+ ATOMIC NUMBER 9 GROUP 7 ELECTRONIC CONFIGURATION 2, 7 • the second shell is gradually filled up until it has eight electrons

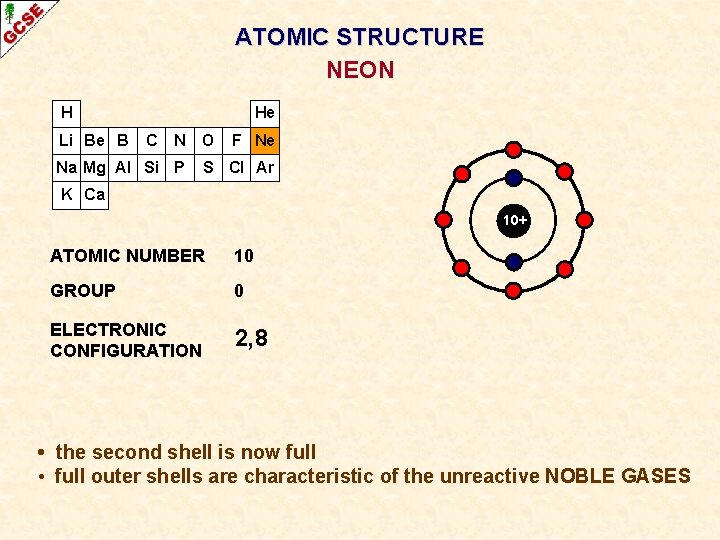
ATOMIC STRUCTURE NEON H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 10+ ATOMIC NUMBER 10 GROUP 0 ELECTRONIC CONFIGURATION 2, 8 • the second shell is now full • full outer shells are characteristic of the unreactive NOBLE GASES

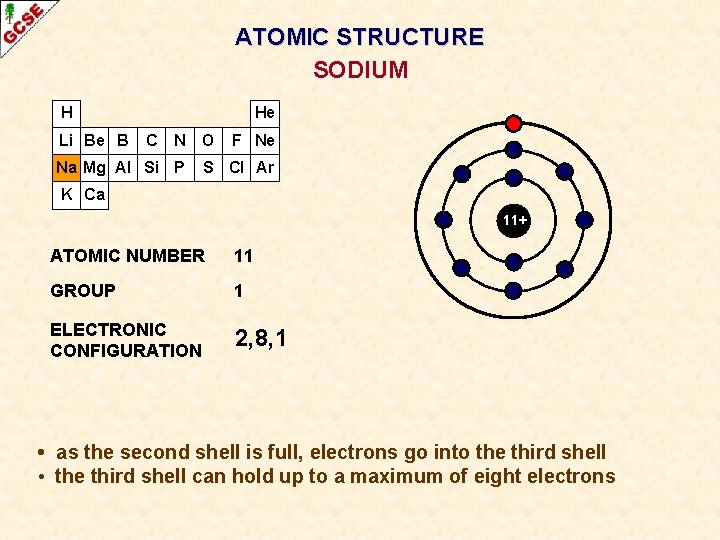
ATOMIC STRUCTURE SODIUM H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 11+ ATOMIC NUMBER 11 GROUP 1 ELECTRONIC CONFIGURATION 2, 8, 1 • as the second shell is full, electrons go into the third shell • the third shell can hold up to a maximum of eight electrons

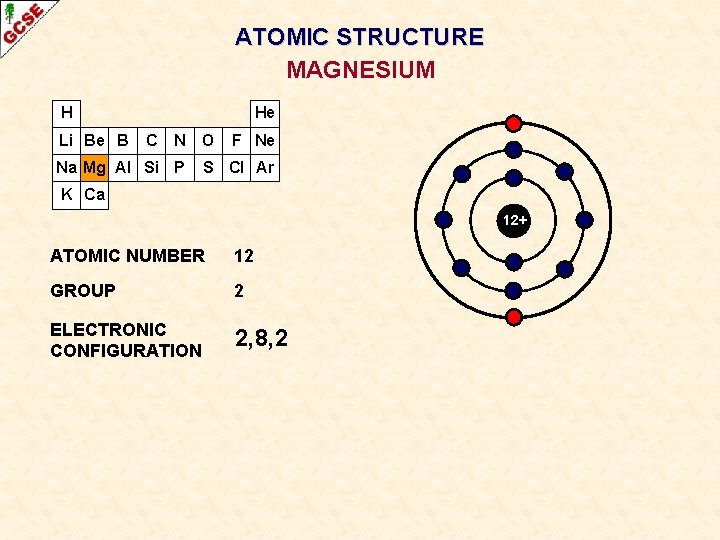
ATOMIC STRUCTURE MAGNESIUM H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 12+ ATOMIC NUMBER 12 GROUP 2 ELECTRONIC CONFIGURATION 2, 8, 2

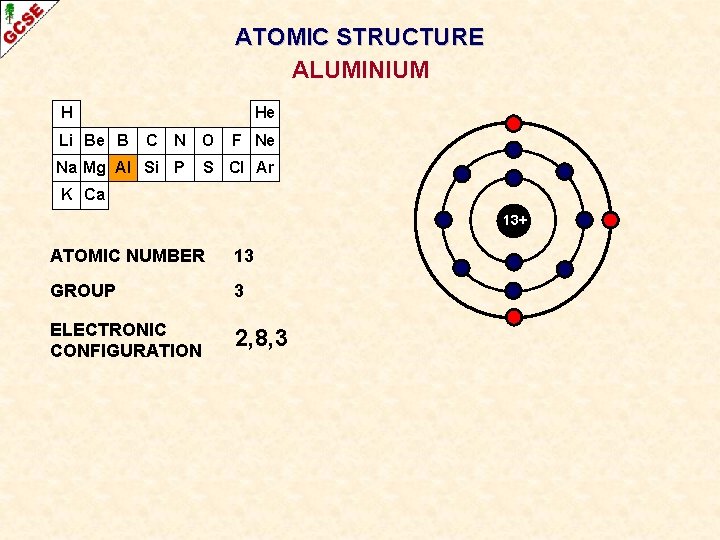
ATOMIC STRUCTURE ALUMINIUM H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 13+ ATOMIC NUMBER 13 GROUP 3 ELECTRONIC CONFIGURATION 2, 8, 3

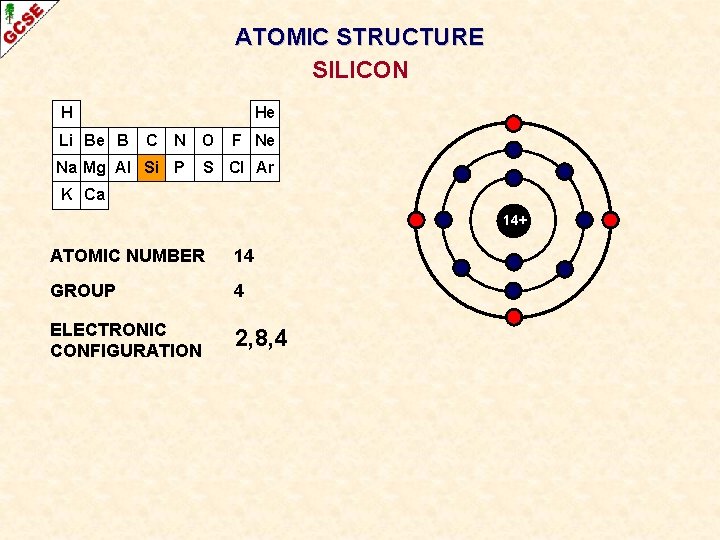
ATOMIC STRUCTURE SILICON H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 14+ ATOMIC NUMBER 14 GROUP 4 ELECTRONIC CONFIGURATION 2, 8, 4

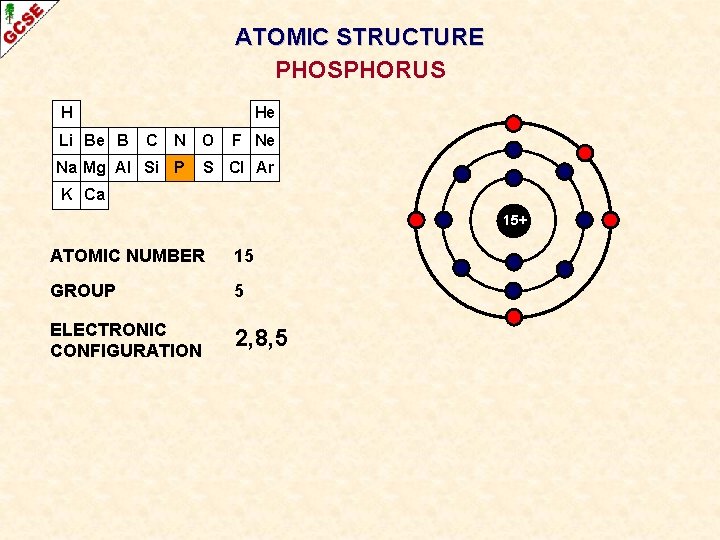
ATOMIC STRUCTURE PHOSPHORUS H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 15+ ATOMIC NUMBER 15 GROUP 5 ELECTRONIC CONFIGURATION 2, 8, 5

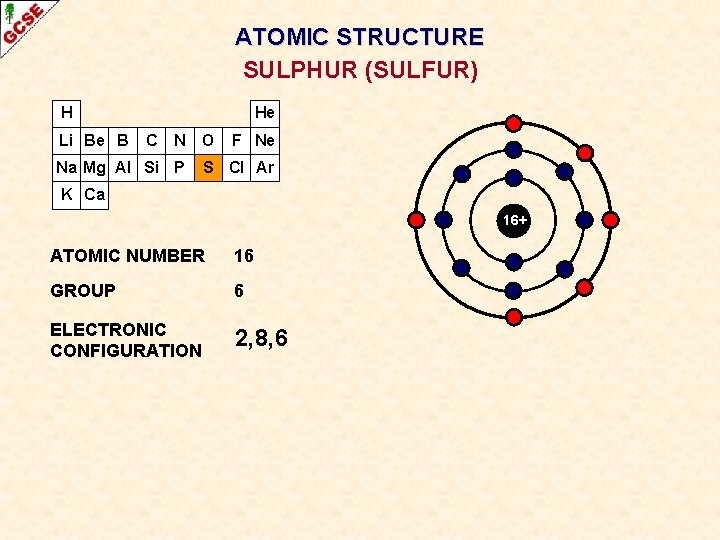
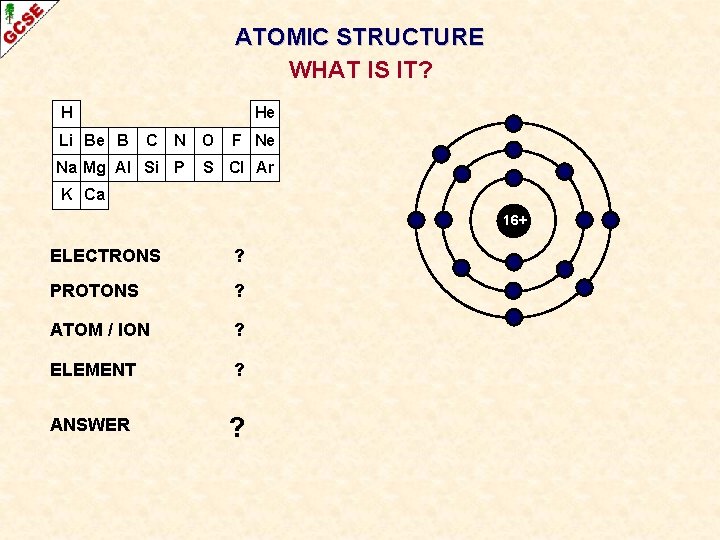
ATOMIC STRUCTURE SULPHUR (SULFUR) H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 16+ ATOMIC NUMBER 16 GROUP 6 ELECTRONIC CONFIGURATION 2, 8, 6

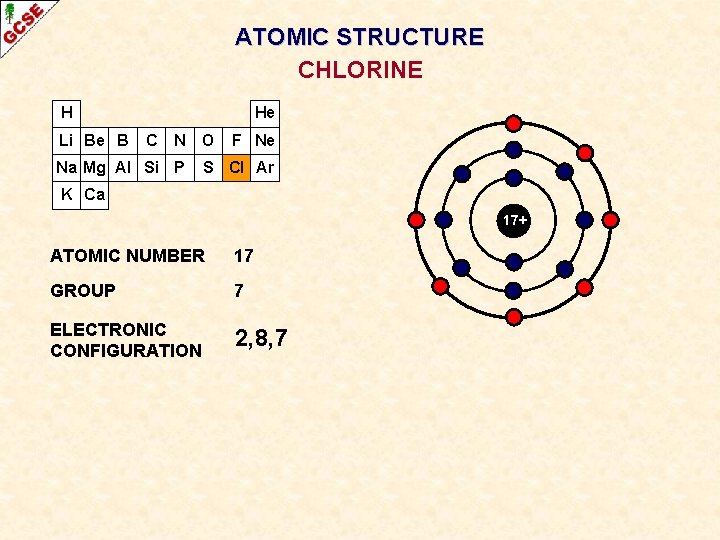
ATOMIC STRUCTURE CHLORINE H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 17+ ATOMIC NUMBER 17 GROUP 7 ELECTRONIC CONFIGURATION 2, 8, 7

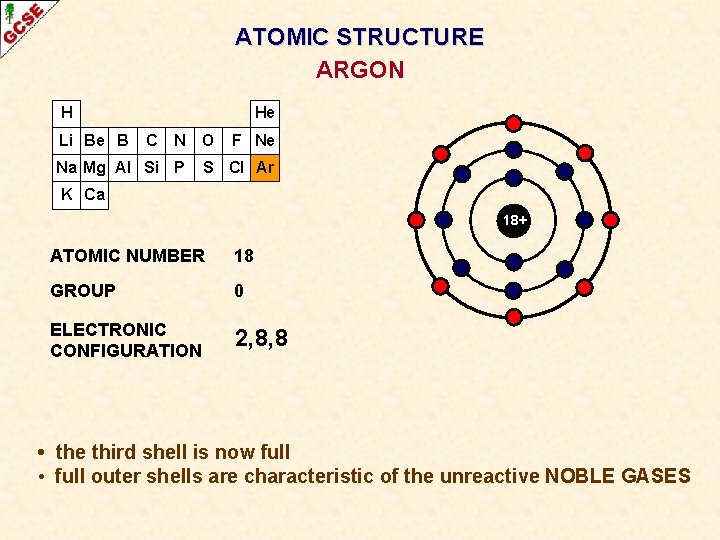
ATOMIC STRUCTURE ARGON H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 18+ ATOMIC NUMBER 18 GROUP 0 ELECTRONIC CONFIGURATION 2, 8, 8 • the third shell is now full • full outer shells are characteristic of the unreactive NOBLE GASES

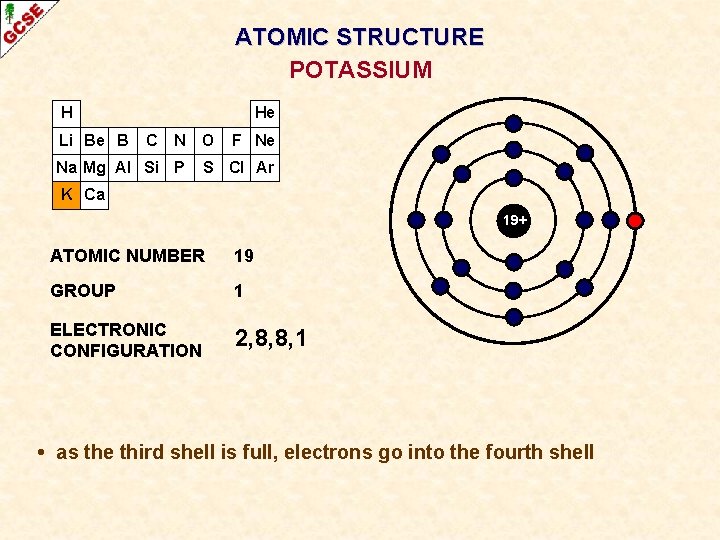
ATOMIC STRUCTURE POTASSIUM H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 19+ ATOMIC NUMBER 19 GROUP 1 ELECTRONIC CONFIGURATION 2, 8, 8, 1 • as the third shell is full, electrons go into the fourth shell

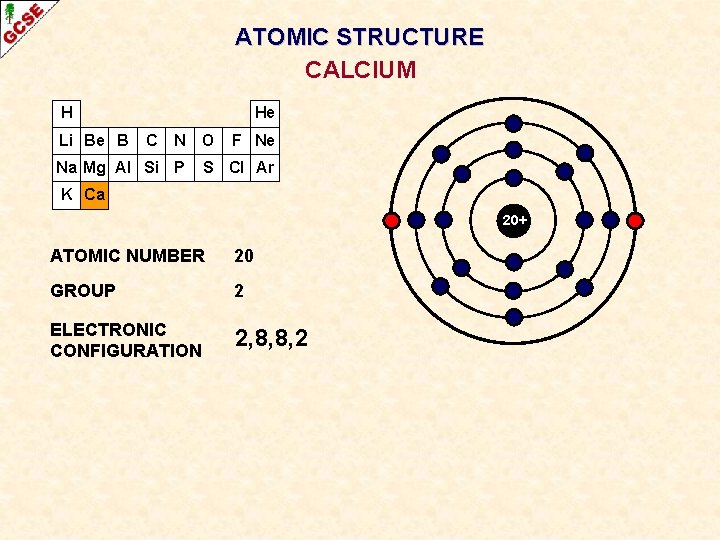
ATOMIC STRUCTURE CALCIUM H Li Be B He C N O Na Mg Al Si P F Ne S Cl Ar K Ca 20+ ATOMIC NUMBER 20 GROUP 2 ELECTRONIC CONFIGURATION 2, 8, 8, 2

ATOMIC STRUCTURE WHAT IS IT?

ATOMIC STRUCTURE WHAT IS IT? H Li Be B He C N Na Mg Al Si P O F Ne S Cl Ar K Ca 16+ ELECTRONS ? PROTONS ? ATOM / ION ? ELEMENT ? ANSWER ?

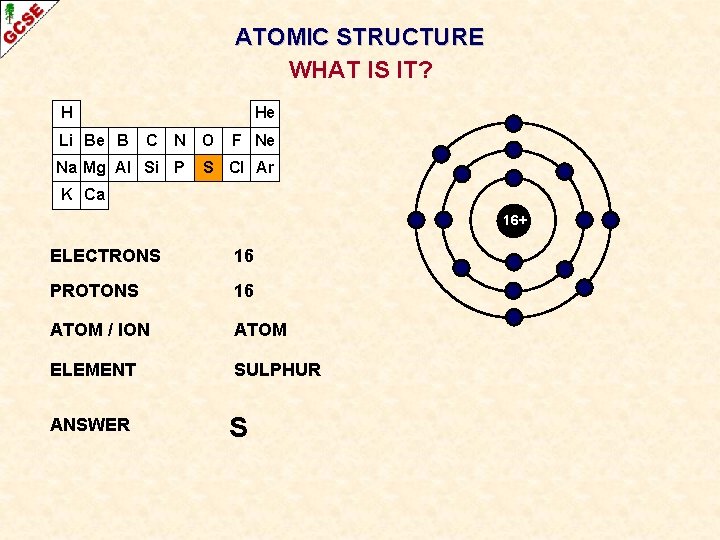
ATOMIC STRUCTURE WHAT IS IT? H Li Be B He C N Na Mg Al Si P O F Ne S Cl Ar K Ca 16+ ELECTRONS 16 PROTONS 16 ATOM / ION ATOM ELEMENT SULPHUR ANSWER S

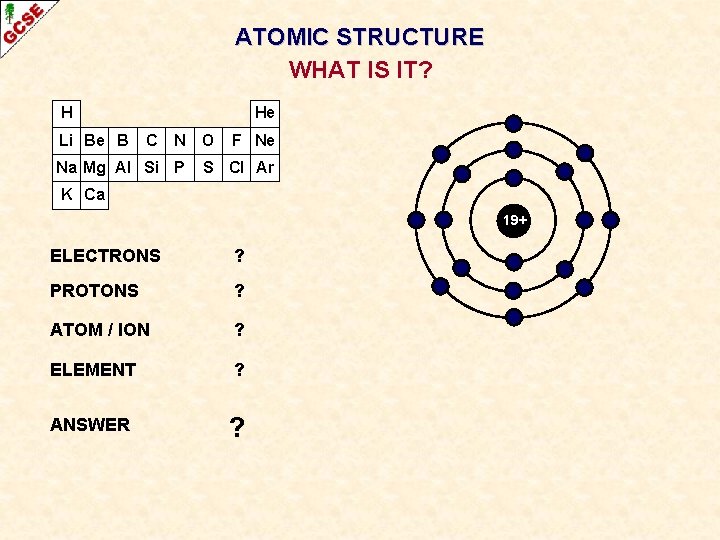
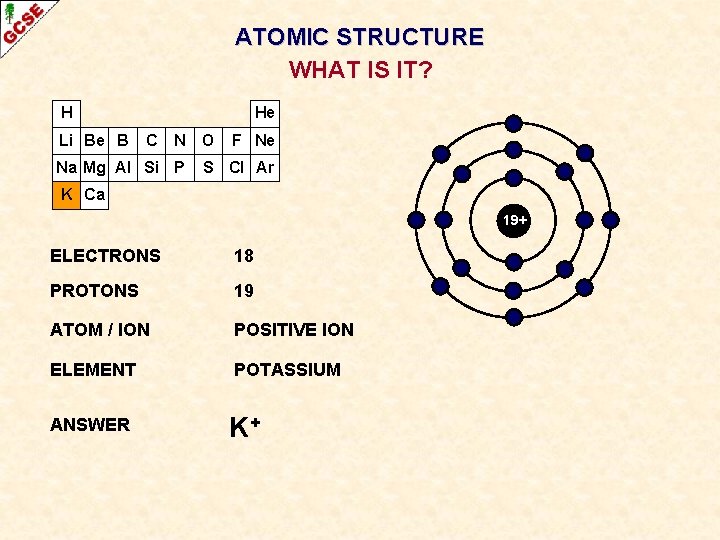
ATOMIC STRUCTURE WHAT IS IT? H Li Be B He C N Na Mg Al Si P O F Ne S Cl Ar K Ca 19+ ELECTRONS ? PROTONS ? ATOM / ION ? ELEMENT ? ANSWER ?

ATOMIC STRUCTURE WHAT IS IT? H Li Be B He C N Na Mg Al Si P O F Ne S Cl Ar K Ca 19+ ELECTRONS 18 PROTONS 19 ATOM / ION POSITIVE ION ELEMENT POTASSIUM ANSWER K+

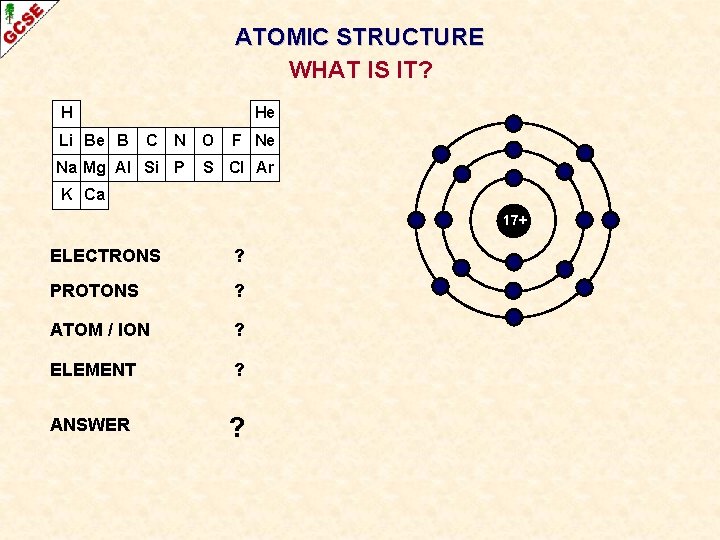
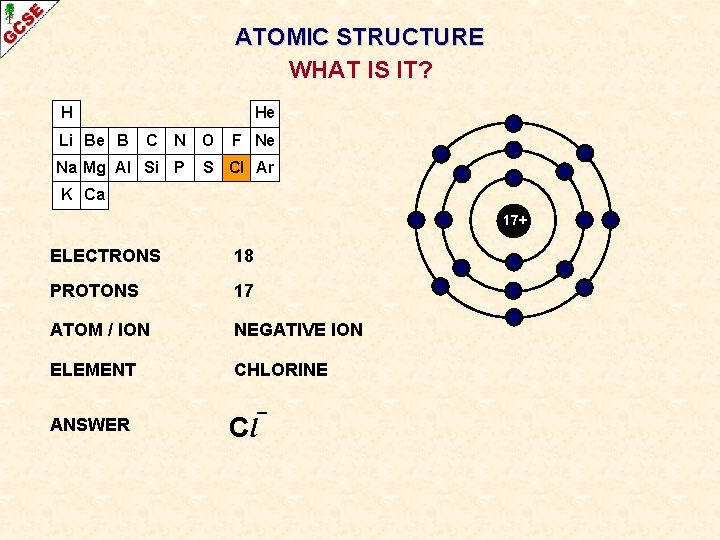
ATOMIC STRUCTURE WHAT IS IT? H Li Be B He C N Na Mg Al Si P O F Ne S Cl Ar K Ca 17+ ELECTRONS ? PROTONS ? ATOM / ION ? ELEMENT ? ANSWER ?

ATOMIC STRUCTURE WHAT IS IT? H Li Be B He C N Na Mg Al Si P O F Ne S Cl Ar K Ca 17+ ELECTRONS 18 PROTONS 17 ATOM / ION NEGATIVE ION ELEMENT CHLORINE ANSWER Cl¯

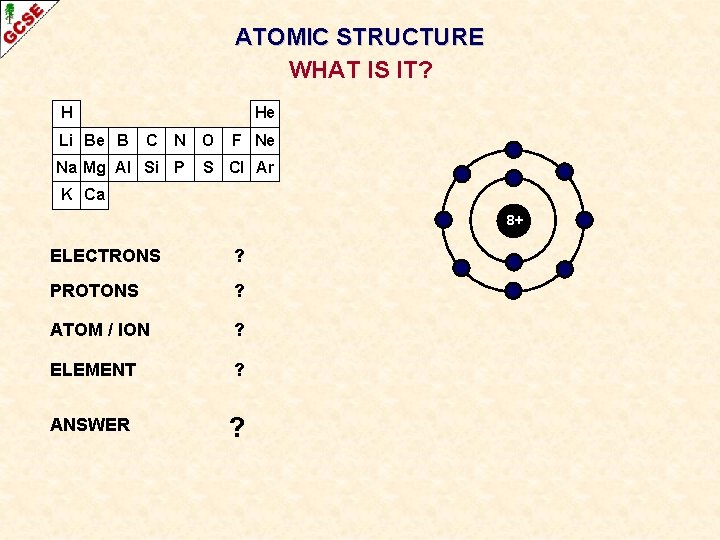
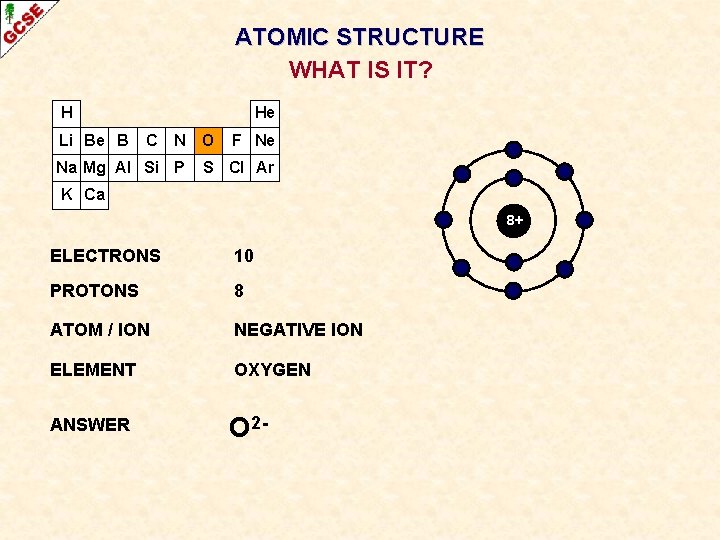
ATOMIC STRUCTURE WHAT IS IT? H Li Be B He C N Na Mg Al Si P O F Ne S Cl Ar K Ca 8+ ELECTRONS ? PROTONS ? ATOM / ION ? ELEMENT ? ANSWER ?

ATOMIC STRUCTURE WHAT IS IT? H Li Be B He C N Na Mg Al Si P O F Ne S Cl Ar K Ca 8+ ELECTRONS 10 PROTONS 8 ATOM / ION NEGATIVE ION ELEMENT OXYGEN ANSWER O 2 -

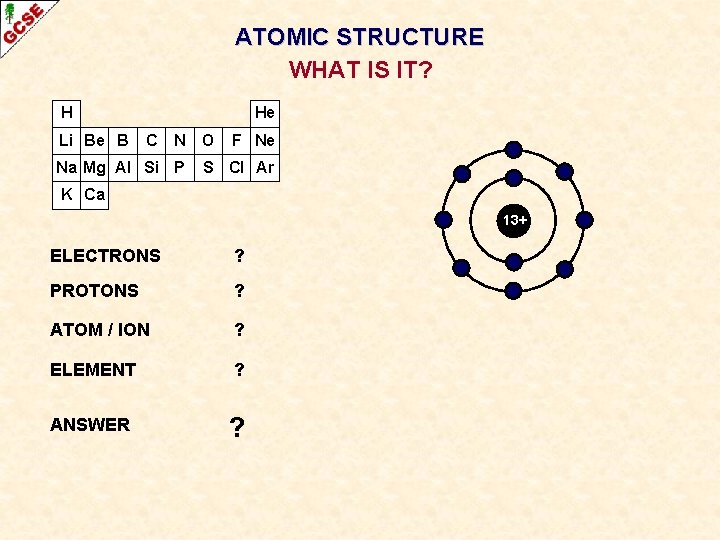
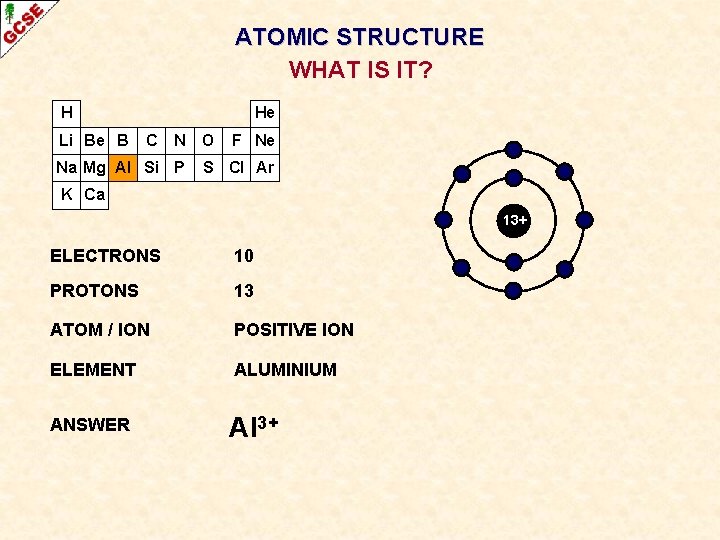
ATOMIC STRUCTURE WHAT IS IT? H Li Be B He C N Na Mg Al Si P O F Ne S Cl Ar K Ca 13+ ELECTRONS ? PROTONS ? ATOM / ION ? ELEMENT ? ANSWER ?

ATOMIC STRUCTURE WHAT IS IT? H Li Be B He C N Na Mg Al Si P O F Ne S Cl Ar K Ca 13+ ELECTRONS 10 PROTONS 13 ATOM / ION POSITIVE ION ELEMENT ALUMINIUM ANSWER Al 3+

ATOMIC STRUCTURE THE END © 2011 JONATHAN HOPTON & KNOCKHARDY PUBLISHING