Atomic Radius Definition Approximate distance from the nucleus







- Slides: 32

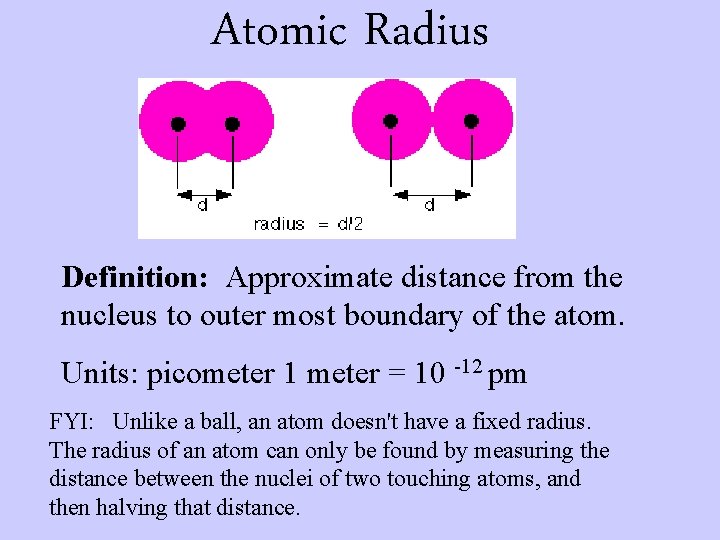
Atomic Radius Definition: Approximate distance from the nucleus to outer most boundary of the atom. Units: picometer 1 meter = 10 -12 pm FYI: Unlike a ball, an atom doesn't have a fixed radius. The radius of an atom can only be found by measuring the distance between the nuclei of two touching atoms, and then halving that distance.

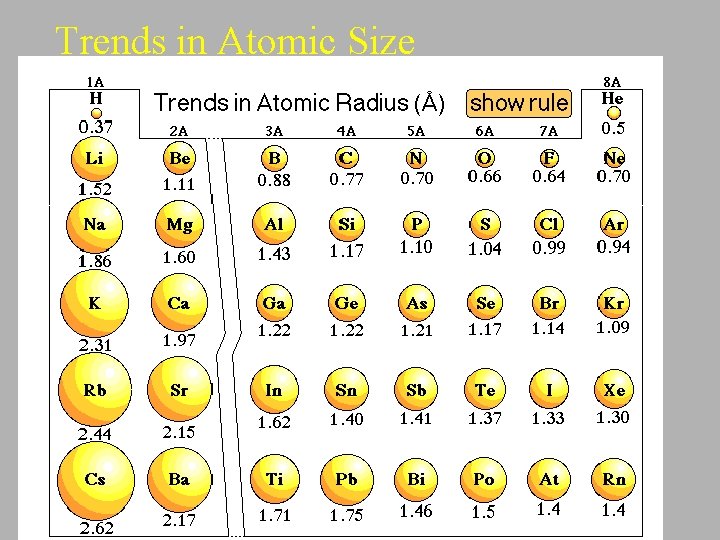
Trends in Atomic Size

Trend in Atomic Size Across a Period Trend: • Atoms get smaller as you go across a period from left to right. Explanation: • Atoms have the same number of shells in a period. • The force of nuclear attraction for valence electrons increases due to the increase in the number of protons. Therefore the valence shells get pulled in making the atom smaller.

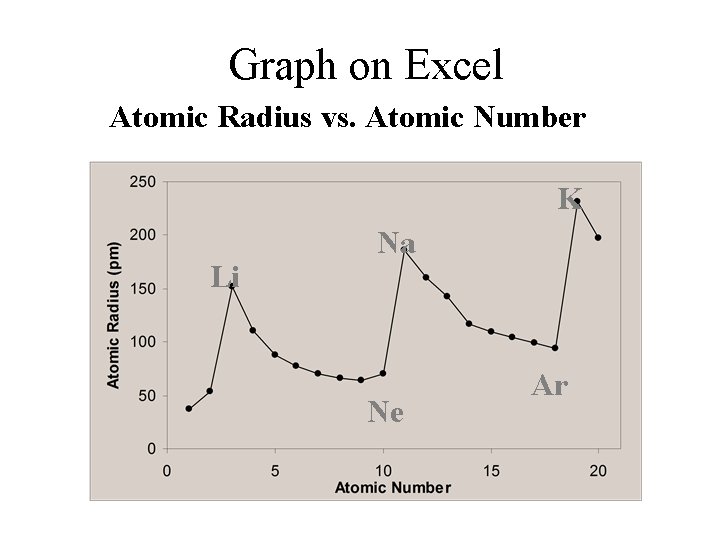
Graph on Excel Atomic Radius vs. Atomic Number K Li Na Ne Ar

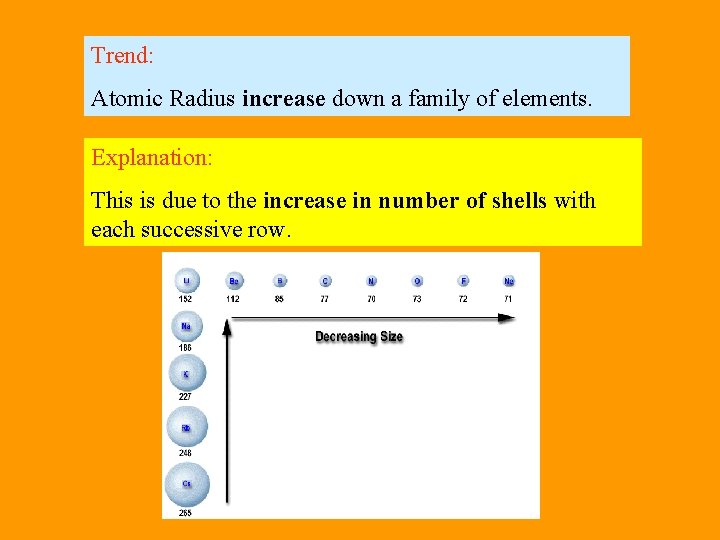
Trend: Atomic Radius increase down a family of elements. Explanation: This is due to the increase in number of shells with each successive row.

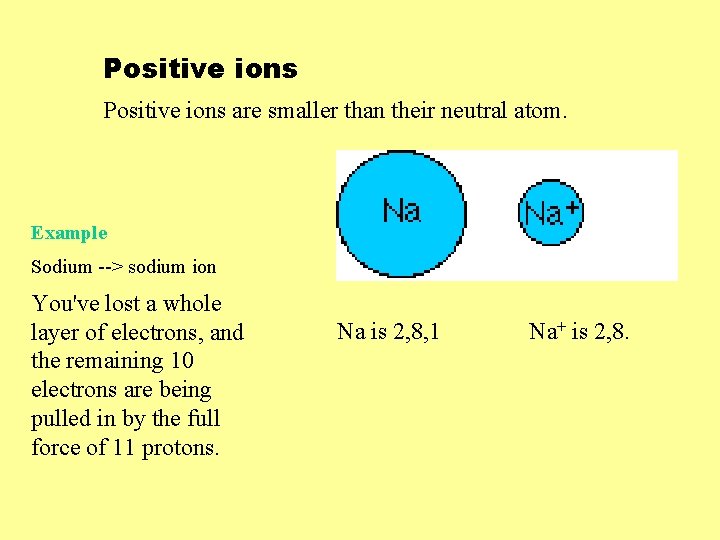
Positive ions are smaller than their neutral atom. Example Sodium --> sodium ion You've lost a whole layer of electrons, and the remaining 10 electrons are being pulled in by the full force of 11 protons. Na is 2, 8, 1 Na+ is 2, 8.

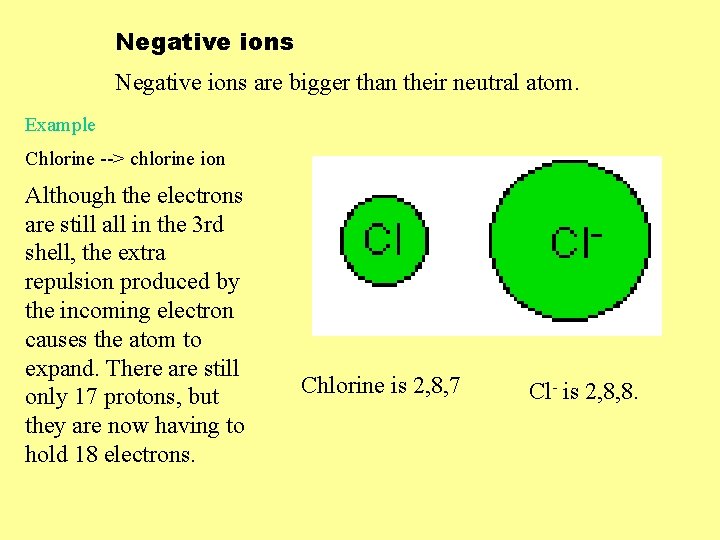
Negative ions are bigger than their neutral atom. Example Chlorine --> chlorine ion Although the electrons are still all in the 3 rd shell, the extra repulsion produced by the incoming electron causes the atom to expand. There are still only 17 protons, but they are now having to hold 18 electrons. Chlorine is 2, 8, 7 Cl- is 2, 8, 8.

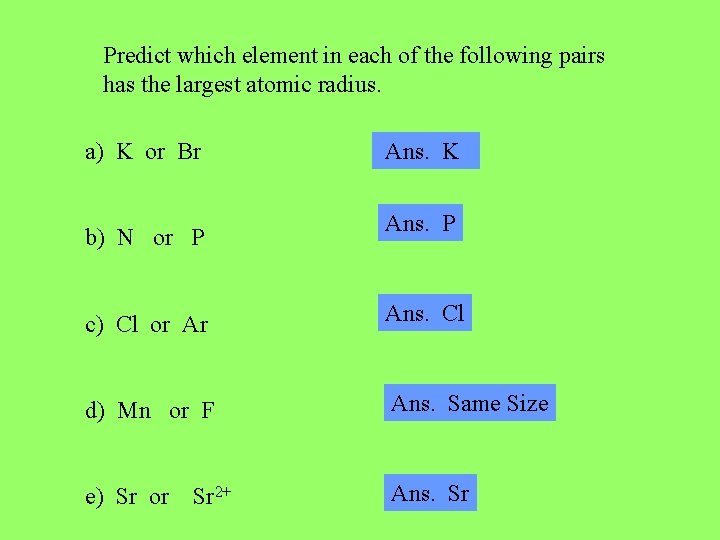
Predict which element in each of the following pairs has the largest atomic radius. a) K or Br b) N or P Ans. K Ans. P c) Cl or Ar Ans. Cl d) Mn or F Ans. Same Size e) Sr or Ans. Sr Sr 2+

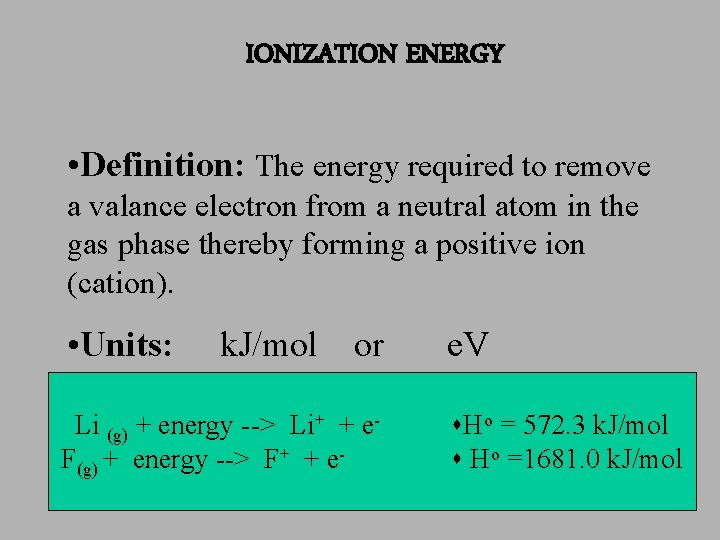
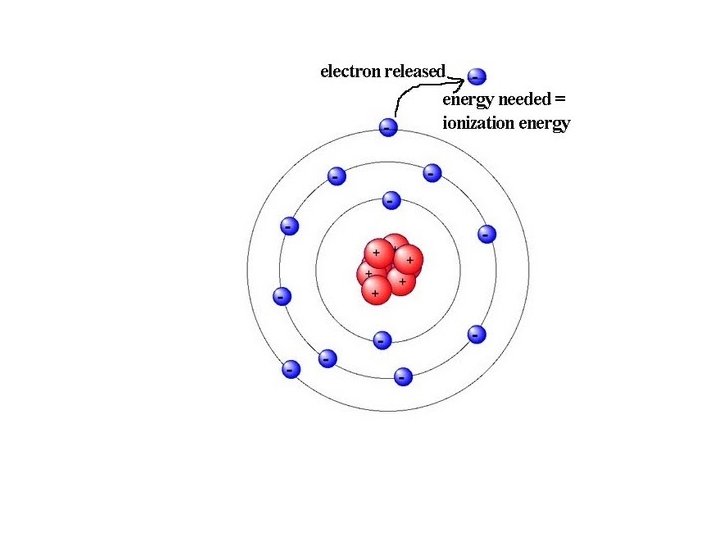
IONIZATION ENERGY • Definition: The energy required to remove a valance electron from a neutral atom in the gas phase thereby forming a positive ion (cation). • Units: k. J/mol or Li (g) + energy --> Li+ + e. F(g) + energy --> F+ + e- e. V Ho = 572. 3 k. J/mol Ho =1681. 0 k. J/mol


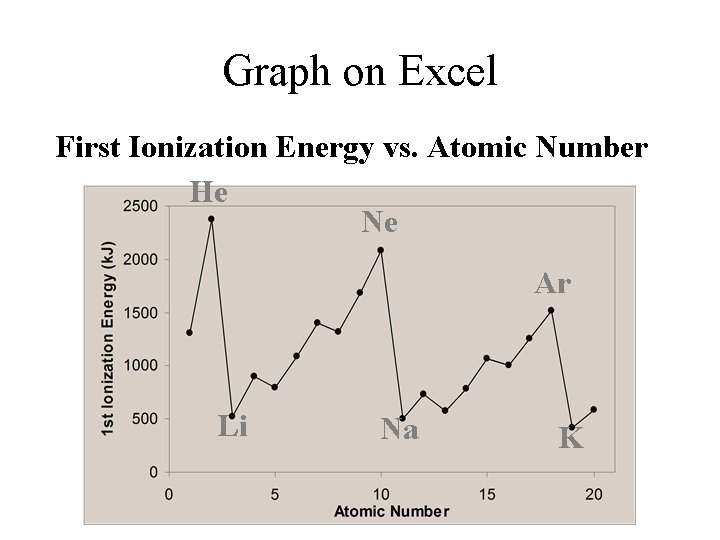
Graph on Excel First Ionization Energy vs. Atomic Number He Ne Ar Li Na K

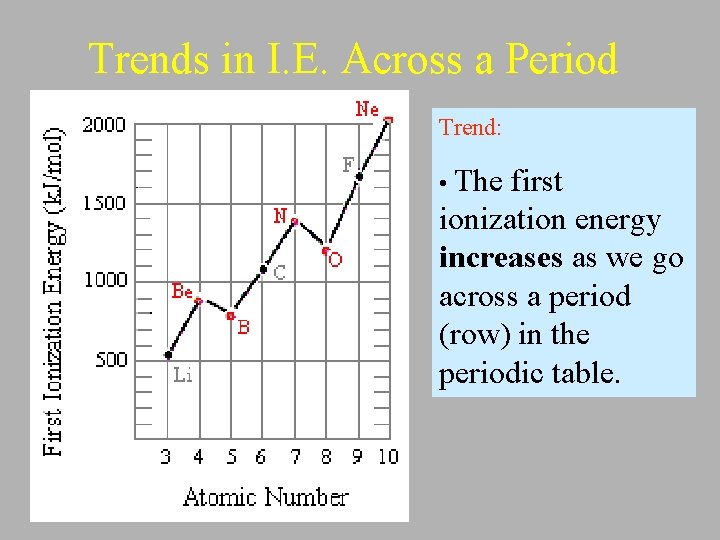
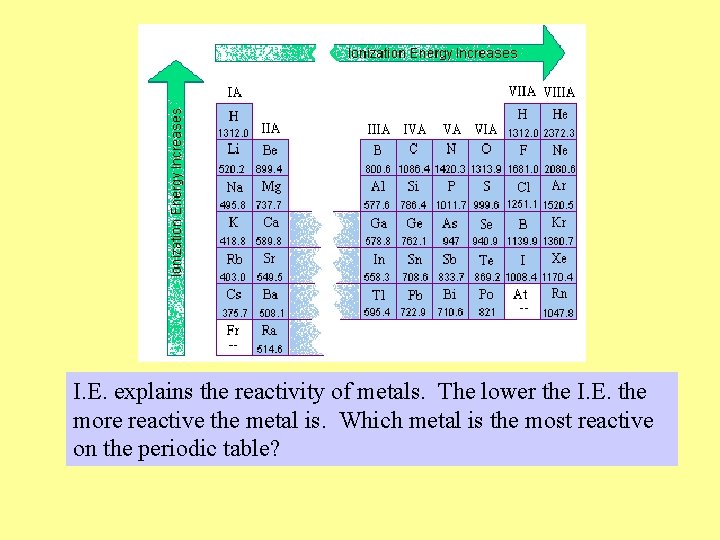
Trends in I. E. Across a Period Trend: • The first ionization energy increases as we go across a period (row) in the periodic table.

Explanation: • There is an increase in the number of protons in nucleus which increases the force of attraction between the nucleus and the valence electrons. • Therefore it is harder to remove an electron and thus requires more energy. • Metals (on the left of P. T. ) tend to have lower I. E. because they give up electrons more easily forming cations.

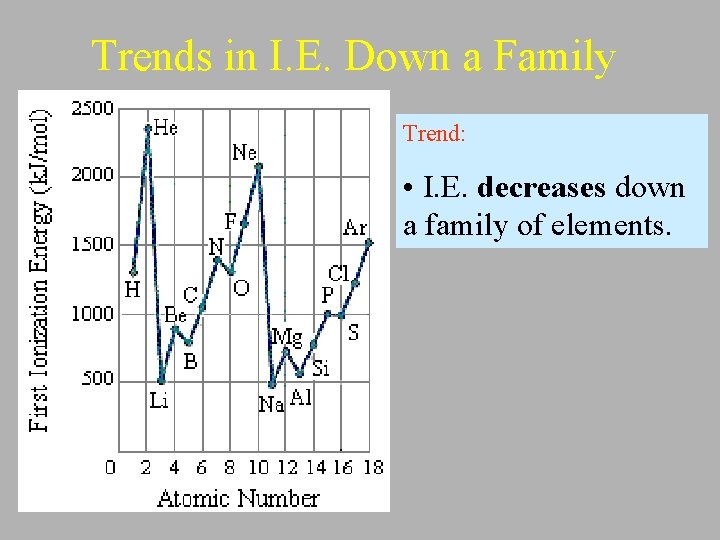
Trends in I. E. Down a Family Trend: • I. E. decreases down a family of elements.

Explanation: • The atom gets larger (1 shell is added) as we go down each successive row in a column of the periodic table. Therefore the valence electron is further away and easier to remove requiring less energy. • Although the number of protons in the nucleus also becomes larger as you move down the column, the electrons in the inner shells shield valence electron from some of the force of attraction of the nucleus.

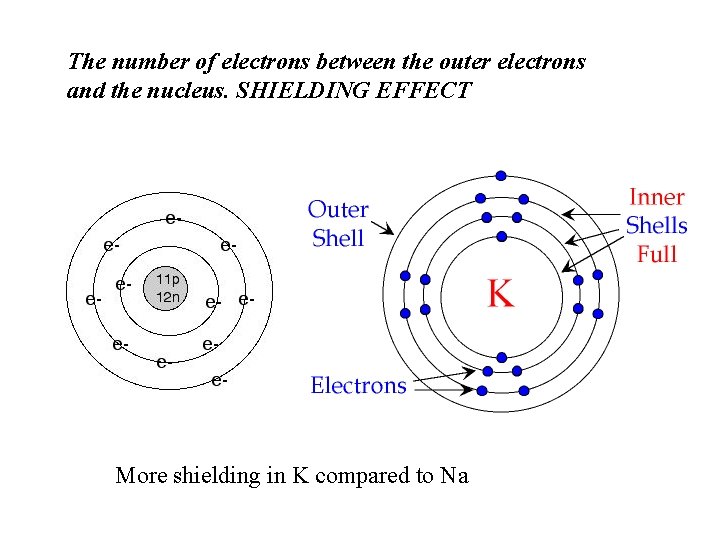
The number of electrons between the outer electrons and the nucleus. SHIELDING EFFECT More shielding in K compared to Na

I. E. explains the reactivity of metals. The lower the I. E. the more reactive the metal is. Which metal is the most reactive on the periodic table?

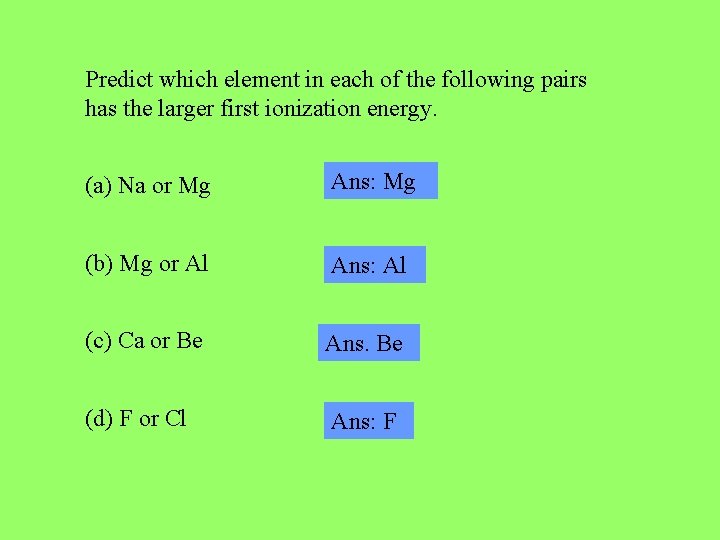
Predict which element in each of the following pairs has the larger first ionization energy. (a) Na or Mg Ans: Mg (b) Mg or Al Ans: Al (c) Ca or Be Ans. Be (d) F or Cl Ans: F

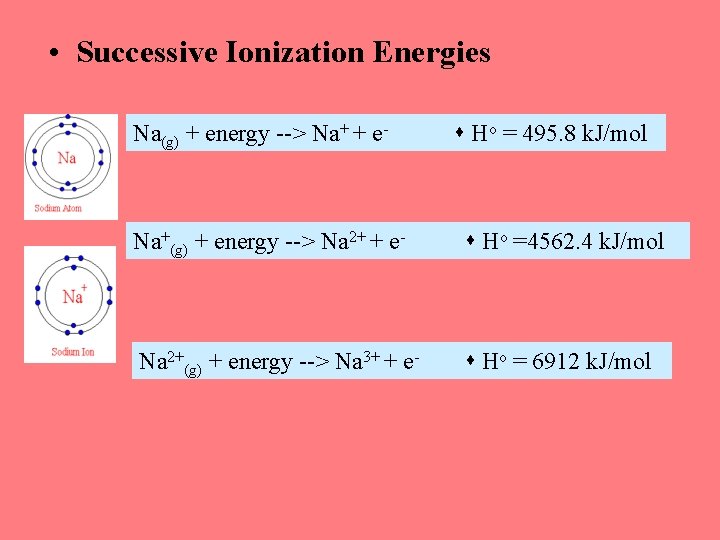
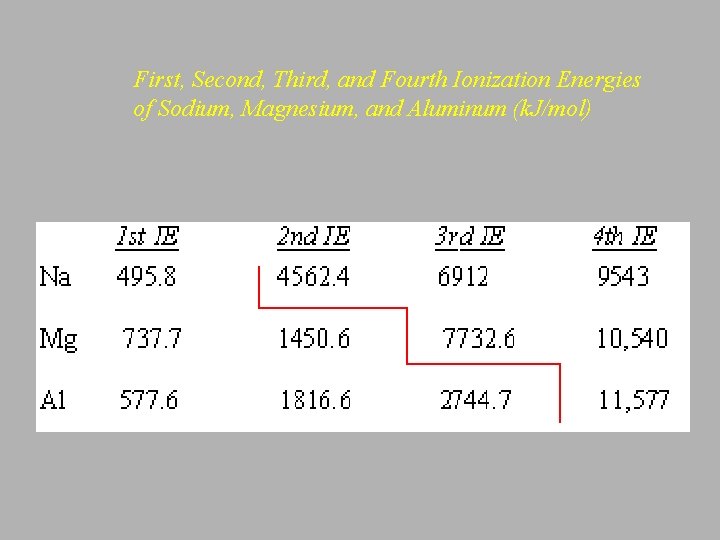
• Successive Ionization Energies Na(g) + energy --> Na+ + e- Ho = 495. 8 k. J/mol Na+(g) + energy --> Na 2+ + e- Ho =4562. 4 k. J/mol Na 2+(g) + energy --> Na 3+ + e- Ho = 6912 k. J/mol

First, Second, Third, and Fourth Ionization Energies of Sodium, Magnesium, and Aluminum (k. J/mol)

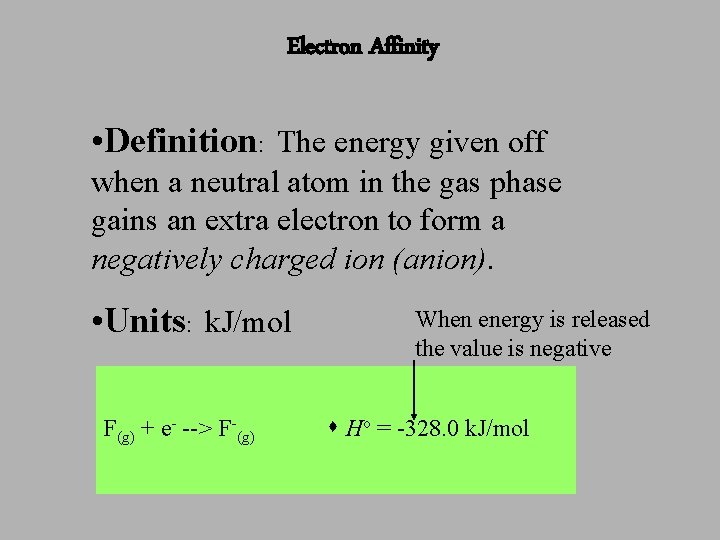
Electron Affinity • Definition: The energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion (anion). • Units: k. J/mol F(g) + e- --> F-(g) When energy is released the value is negative Ho = -328. 0 k. J/mol

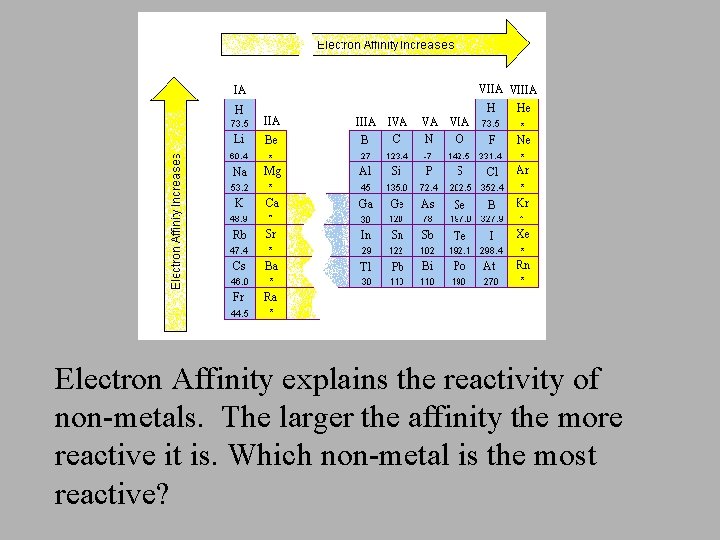
Electron Affinity explains the reactivity of non-metals. The larger the affinity the more reactive it is. Which non-metal is the most reactive?


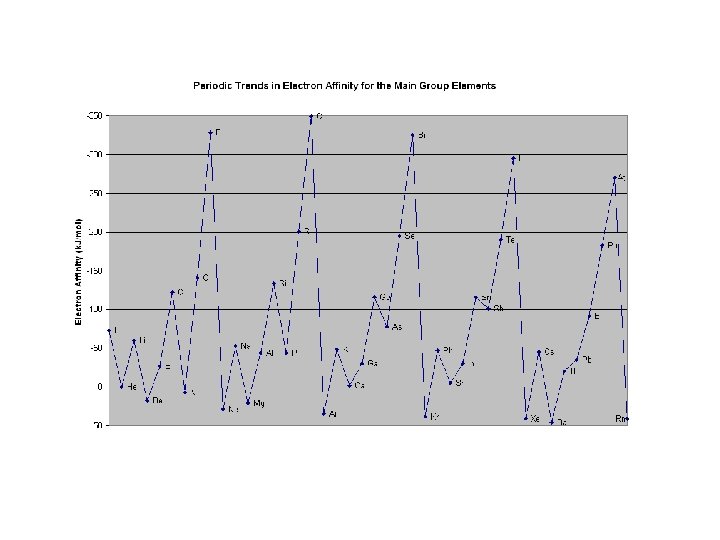
Trend: E. A. increases left to right across a period Explanation: The atom is also getting smaller from left to right therefore the nucleus has a strong attraction for the incoming electron, thus more energy is released. • Elements on the right (non-metals) of the periodic table want electrons to complete their octets by forming anions. Trend: E. A. decreases down a family. Explanation: As you move down a family there are more shells with electrons which tend to repel the one trying to be added, therefore the electron affinity is not as great.

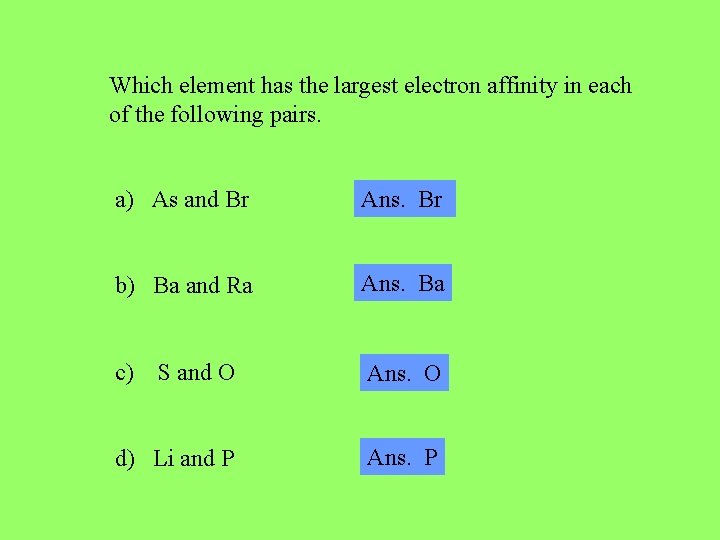
Which element has the largest electron affinity in each of the following pairs. a) As and Br Ans. Br b) Ba and Ra Ans. Ba c) S and O Ans. O d) Li and P Ans. P

Reference pages are 52 - 59 Chemistry 11, Mc. Graw Hill. • Please complete questions 2, 3, 4 on page 60.

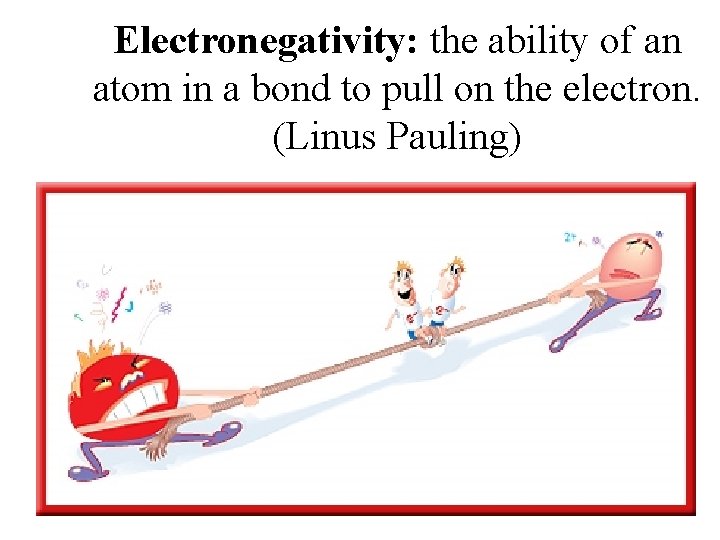
Electronegativity: the ability of an atom in a bond to pull on the electron. (Linus Pauling)

Electronegativity • When electrons are shared by two atoms a covalent bond is formed. • When the atoms are the same they pull on the electrons equally. Example, H-H. • When the atoms are different, the atoms pull on the electrons unevenly. Example, HCl

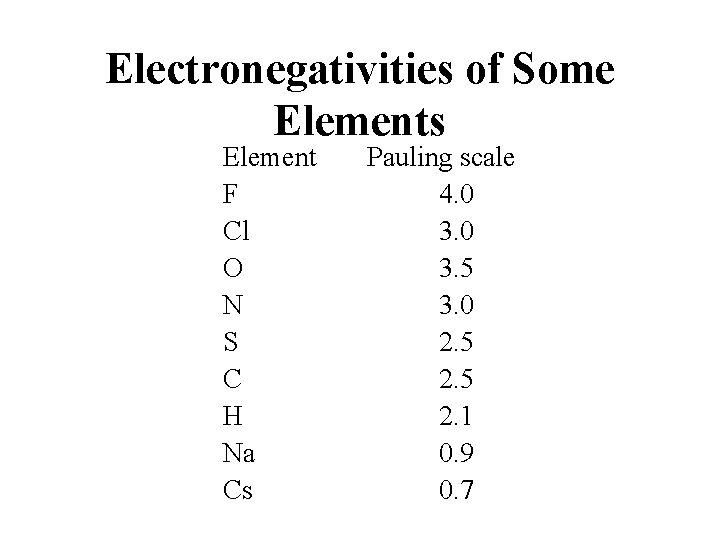
Electronegativities of Some Elements Element F Cl O N S C H Na Cs Pauling scale 4. 0 3. 5 3. 0 2. 5 2. 1 0. 9 0. 7

Trends in Electronegativity • Electronegativity generally decreases as you move down a group. • Electronegativity of the representative elements (Group A elements) increases as you move across a period.

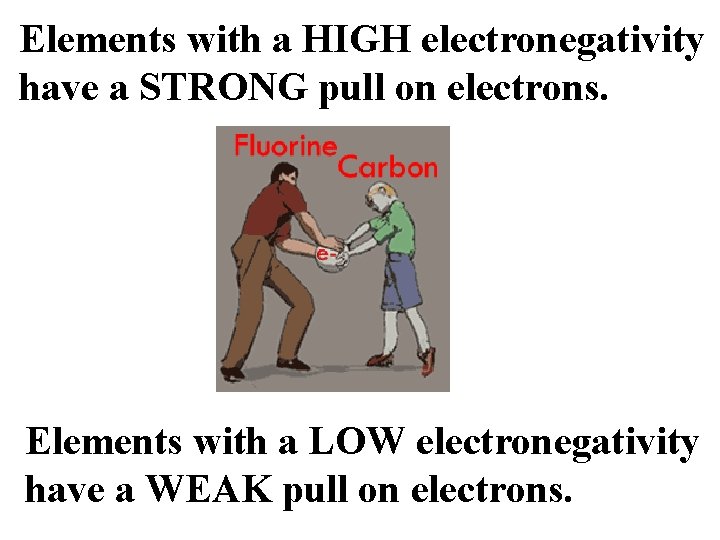
Elements with a HIGH electronegativity have a STRONG pull on electrons. Elements with a LOW electronegativity have a WEAK pull on electrons.

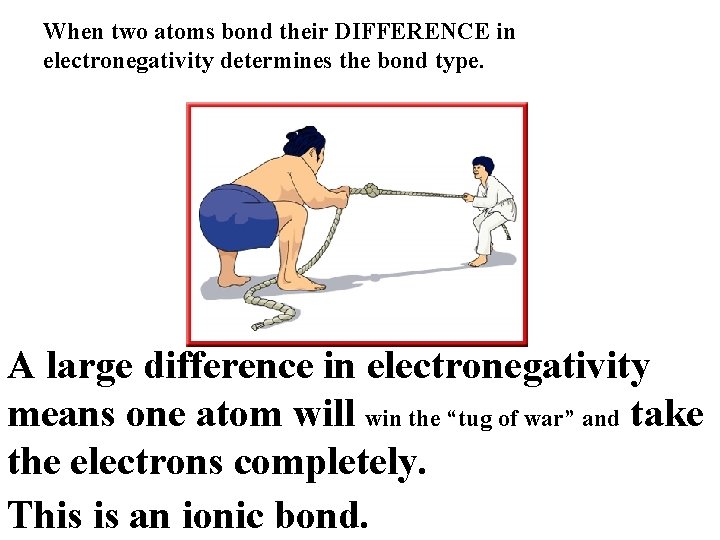
When two atoms bond their DIFFERENCE in electronegativity determines the bond type. difference in electronegativity A large difference in electronegativity means one atom will win the “tug of war” and take the electrons completely. This is an ionic bond.