Atomic Orbitals and Electron Configurations Chap 5 Section

Atomic Orbitals and Electron Configurations (Chap 5, Section 5. 3)
Quantum Mechanics Better than any previous model, quantum mechanics does explain how the atom behaves. Quantum mechanics treats electrons not as particles, but more as waves (like light waves) which can gain or lose energy.
Atomic Orbitals http: //milesmathis. com/bohr 2. jpg Much like the Bohr model, the energy levels in quantum mechanics describe locations where electrons are likely to occur Quantum mechanics calculates the probabilities of the likelihood of finding an electron

Atomic Orbitals are “geometric shapes” around the nucleus where electrons are found. Rule: No more than 2 electrons can ever be in 1 orbital. The orbital just defines an “area” where you can find an electron.

Address of the electron = Quantum numbers Distance from the nucleus and main energy level: Principal quantum number, n = 1, … 9. Shape (s, p, d, f) of the orbitals, sub-levels Position of the orbitals within the main energy level Behaviour of the electron itself: its spin, clockwise or anticlockwise
Energy Sub-levels Each Principal quantum number or the main energy level has 1 or more “sub-levels” which describe the shape of the orbitals. n = 1 has 1 sub-level (the “s” orbital) n = 2 has 2 sub-levels (“s” and “p”) n = 3 has 3 sub-levels (“s”, “p” and “d”) n = 4 has 4 sub-levels (“s”, “p”, “d” and “f”)
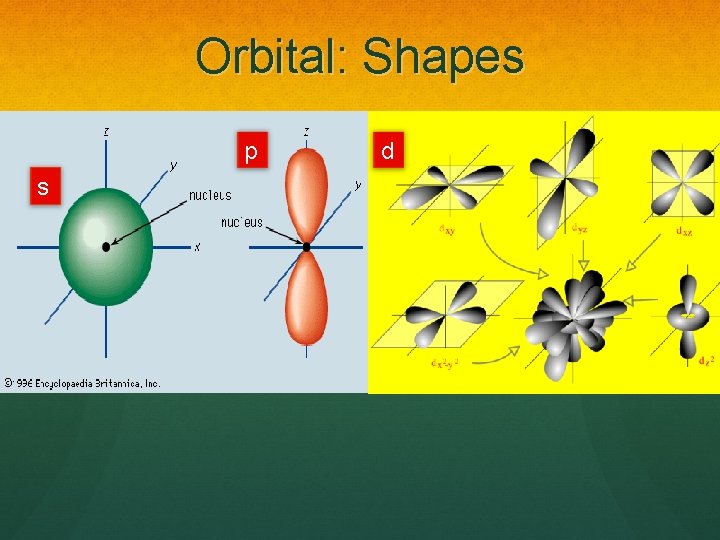
Orbital: Shapes p s d
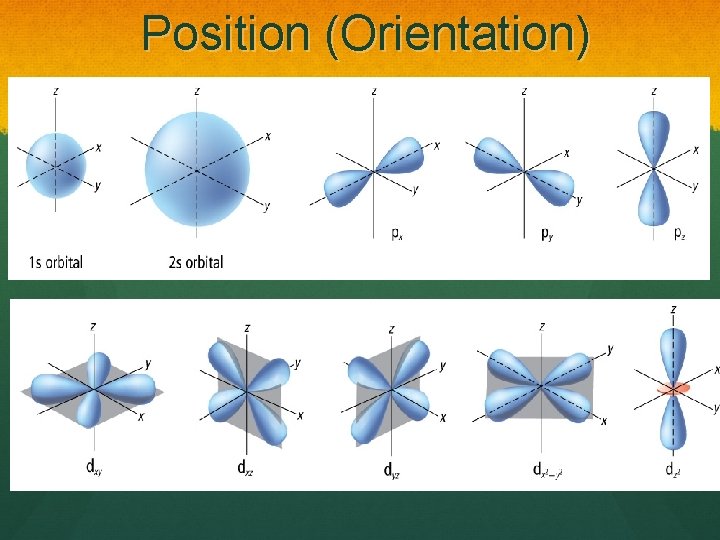
Position (Orientation)
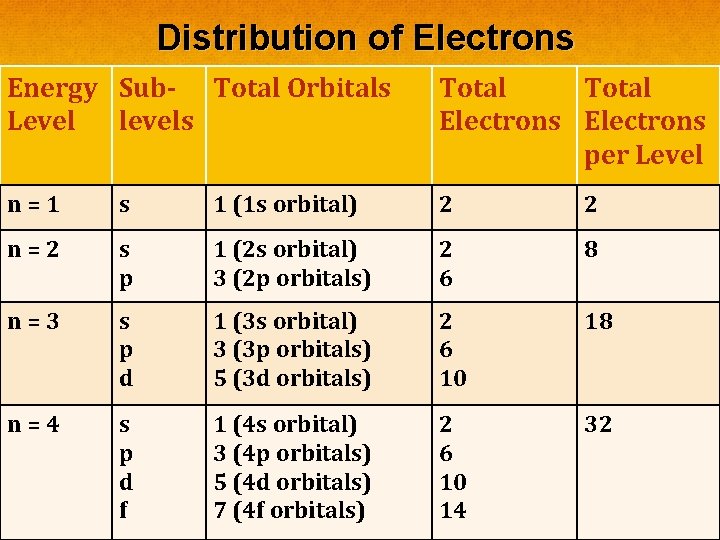
Distribution of Electrons Energy Sub- Total Orbitals Level levels Total Electrons per Level n=1 s 1 (1 s orbital) 2 2 n=2 s p 1 (2 s orbital) 3 (2 p orbitals) 2 6 8 n=3 s 1 (3 s orbital) 2 18 Complete the chartorbitals) in your notes as 6 we discuss this. p 3 (3 p d first level 5 (3 d orbitals) 10 It has only 1. The (n=1) has an s orbital. There are no other orbitals in the first energy level. n=4 s 1 (4 s orbital) 2 32 Wep call this 3 orbital the 1 s orbital. 6 (4 p orbitals) d 5 (4 d orbitals) 10 f 7 (4 f orbitals) 14

Electron Configurations The electron configuration is the specific way in which the atomic orbitals are filled. The address of the electron, electron configuration tells the electrons “live. ”
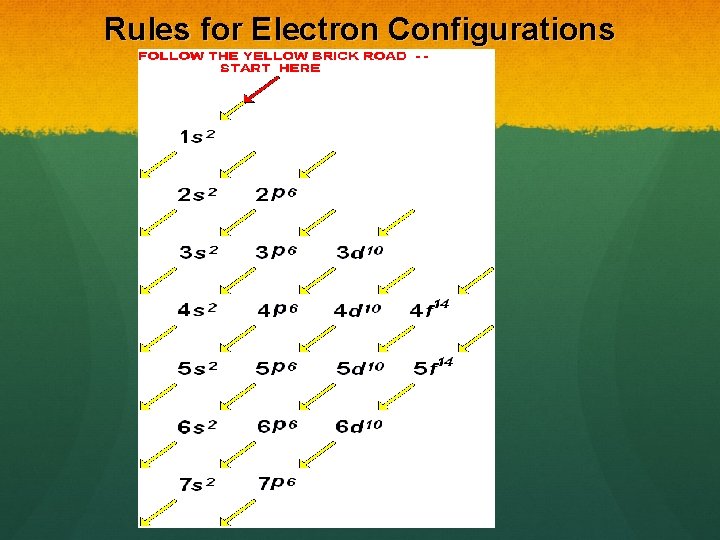
Rules for Electron Configurations

Fill Lower Energy Orbitals FIRST The Aufbau Principle states that electrons enter the lowest energy orbitals first. The lower the principal quantum number (n) the lower the energy.

Aufbau’s Principle Within an energy level, s orbitals are the lowest energy, followed by p, d and then f. f orbitals have the highest energy for that level. s< p< d< f

No more than 2 Electrons in Any Orbital…ever The Pauli Exclusion Principle states that an atomic orbital may have up to 2 electrons. The spins of the electrons must be opposite. We usually represent this with an up arrow and a down arrow.

http: //commonsensequantum. blogspot. co m/2010/10/explaining-electron-spin-andpauli. html
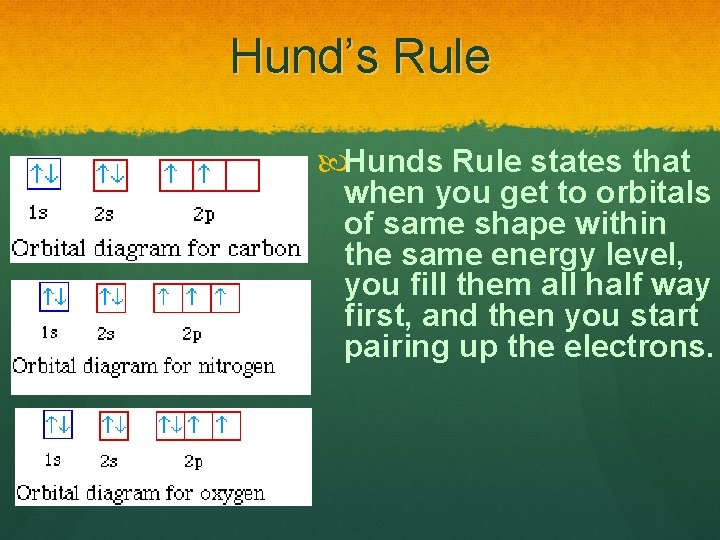
Hund’s Rule Hunds Rule states that when you get to orbitals of same shape within the same energy level, you fill them all half way first, and then you start pairing up the electrons.

Electron Configuration NOW that we know the rules, we can try to write some electron configurations. Remember to use your orbital filling guide to determine WHICH orbital comes next. Lets write some electron configurations for the first few elements, and let’s start with hydrogen.

Electron Configurations Element Configuration H Z=1 1 s 1 He Z=2 1 s 2 Li Z=3 1 s 22 s 1 Be Z=4 1 s 22 s 2 F Z=9 1 s 22 p 5 Ne Z=10 1 s 22 p 6 (2 p is now full) K Z=19 1 s 22 p 63 s 23 p 6 Sc 4 s 1 Z=21 1 s 22 p 63 s 23 p 64 s 23 d 1 Fe Z=26 1 s 22 p 63 s 23 p 6 Br 4 s 23 d 6 Z=35 1 s 22 p 63 s 23 p 64 s 23 d 104 p 5
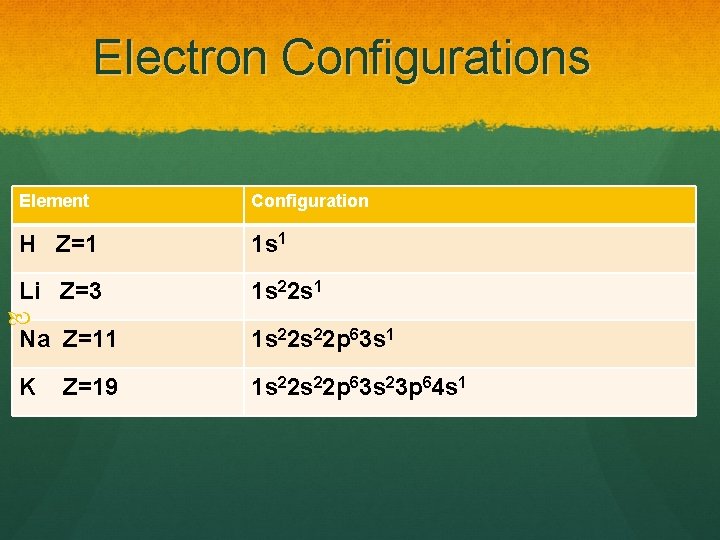
Electron Configurations Element Configuration H Z=1 1 s 1 Li Z=3 Na Z=11 K Z=19 1 s 22 s 1 1 s 22 s 22 p 63 s 23 p 64 s 1

Electron Configuration Chemical Behaviour • . • Electrons in the highest principal energy level are called valence electrons.

Electron Configuration Chemical Behaviour Look at the previous slide and look at just hydrogen, lithium, sodium and potassium. Notice their electron configurations. Do you see any similarities? Since H and Li and Na and K are all in Group 1 A, they all have a similar ending. (s 1)
- Slides: 21