Atomic Nuclear Chemistry Monday 102912 Prep 1 Find











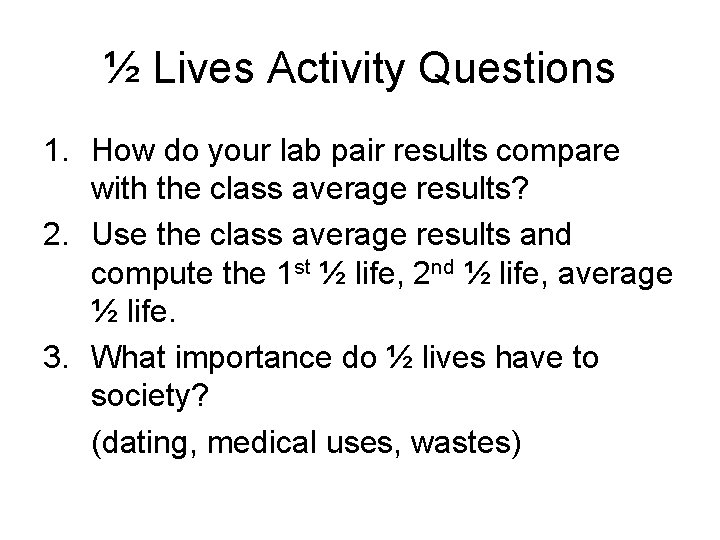

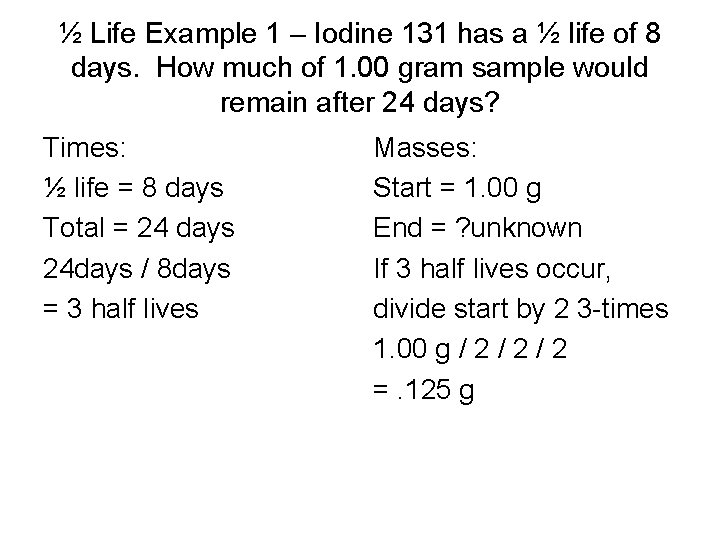




- Slides: 40

Atomic & Nuclear Chemistry

Monday 10/29/12 Prep: 1. Find geiger counter & samples Class: 1. Coop Quiz – A Mole Conversion & An Avg Atomic Mass Problem 2. Isotopes (Quick Review) Radiation Demos 3. Alpha, Beta, Gamma Radiation 4. Fission vs Fusion 5. Nuclear Equations – Alpha, Beta Asmt: Write Nuclear Equations for the Uranium Decay Series

Vocabulary 2 B (Define in your notebook) Isotope Radioisotope Radioactivity Radiation Fission Fusion Radioactive decay Alpha particle Beta particle Gamma ray Nuclear chain reaction Dosimeter nuclear reactor nuclear weapon half life nuclear equation positron radiocarbon dating critical mass nuclear bombardment curie rem plasma transmutation

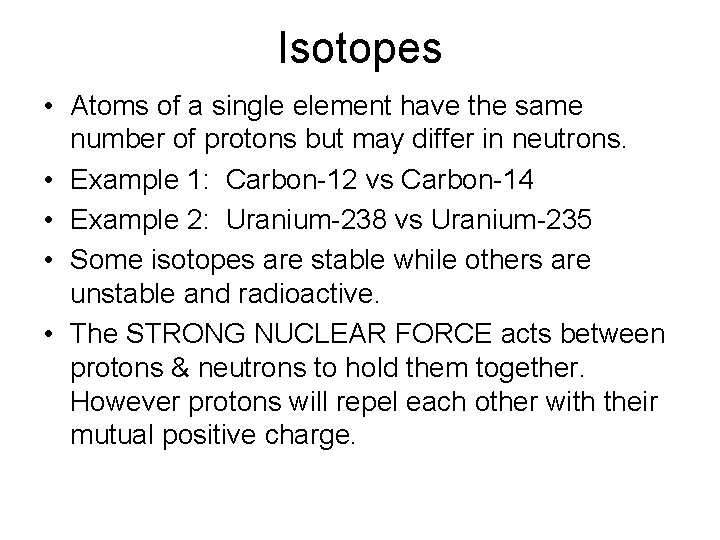
Isotopes • Atoms of a single element have the same number of protons but may differ in neutrons. • Example 1: Carbon-12 vs Carbon-14 • Example 2: Uranium-238 vs Uranium-235 • Some isotopes are stable while others are unstable and radioactive. • The STRONG NUCLEAR FORCE acts between protons & neutrons to hold them together. However protons will repel each other with their mutual positive charge.

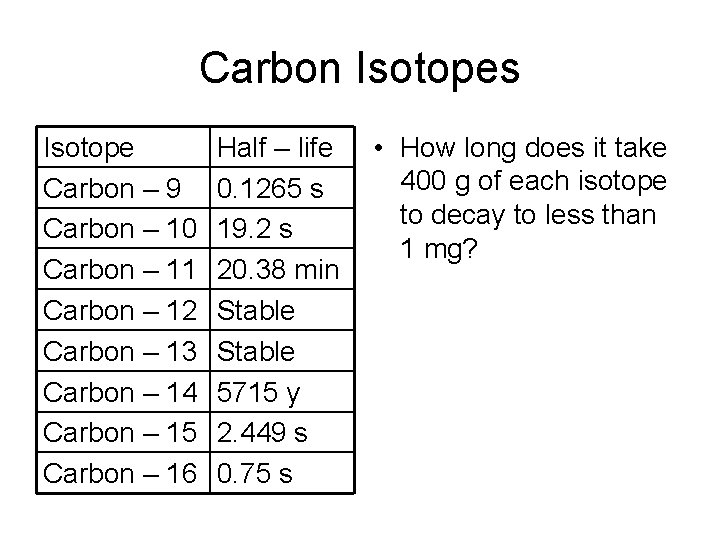
Carbon Isotopes Isotope Carbon – 9 Carbon – 10 Carbon – 11 Carbon – 12 Carbon – 13 Carbon – 14 Carbon – 15 Carbon – 16 Half – life 0. 1265 s 19. 2 s 20. 38 min Stable 5715 y 2. 449 s 0. 75 s • How long does it take 400 g of each isotope to decay to less than 1 mg?

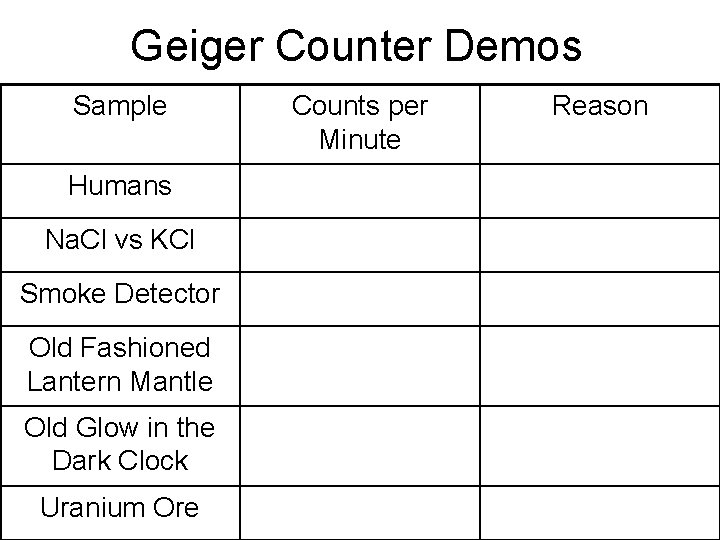
Geiger Counter Demos Sample Humans Na. Cl vs KCl Smoke Detector Old Fashioned Lantern Mantle Old Glow in the Dark Clock Uranium Ore Counts per Minute Reason

Becquerel/Curries • Becquerel – First observed photographic evidence of radioactivity • Curie – Discovered radioactive elements of radium and polonium • Curie – made gift of 1 gram of Radium to President Truman to help fight cancer.

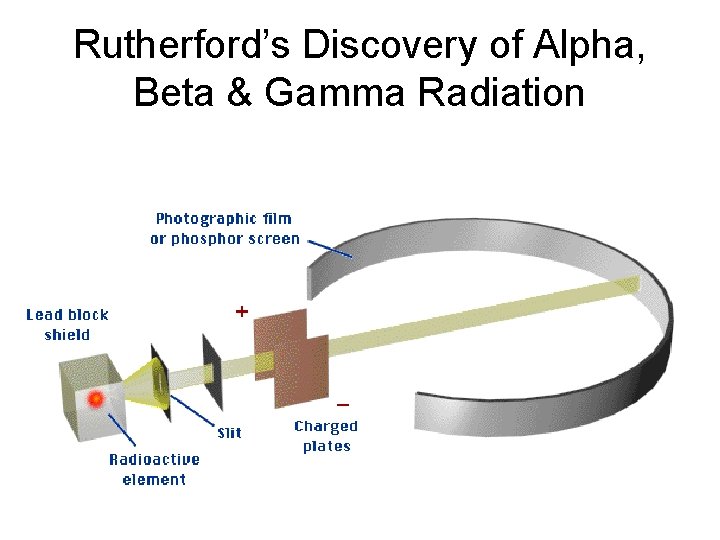
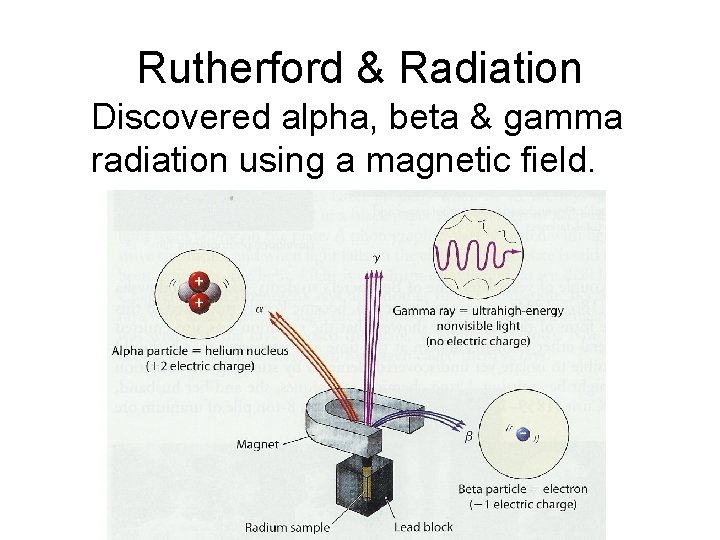
Rutherford’s Discovery of Alpha, Beta & Gamma Radiation

Rutherford & Radiation Discovered alpha, beta & gamma radiation using a magnetic field.

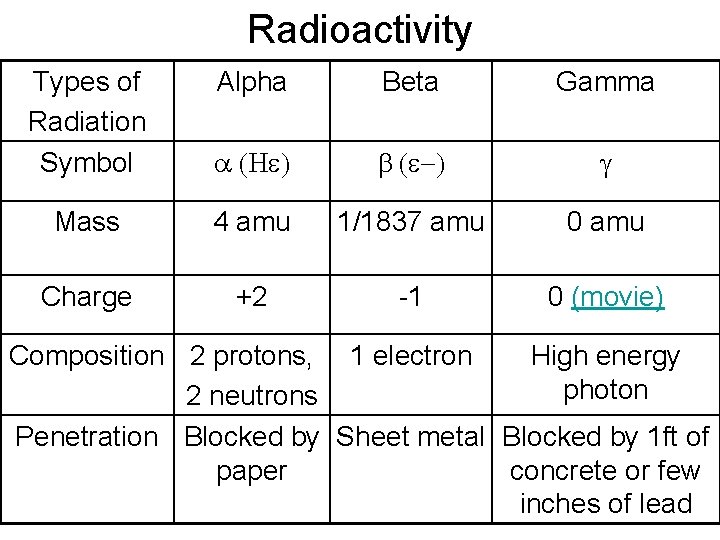
Radioactivity Types of Radiation Symbol Alpha Beta Gamma a (He) b (e-) g Mass 4 amu 1/1837 amu 0 amu Charge +2 -1 0 (movie) Composition 2 protons, 1 electron High energy photon 2 neutrons Penetration Blocked by Sheet metal Blocked by 1 ft of paper concrete or few inches of lead

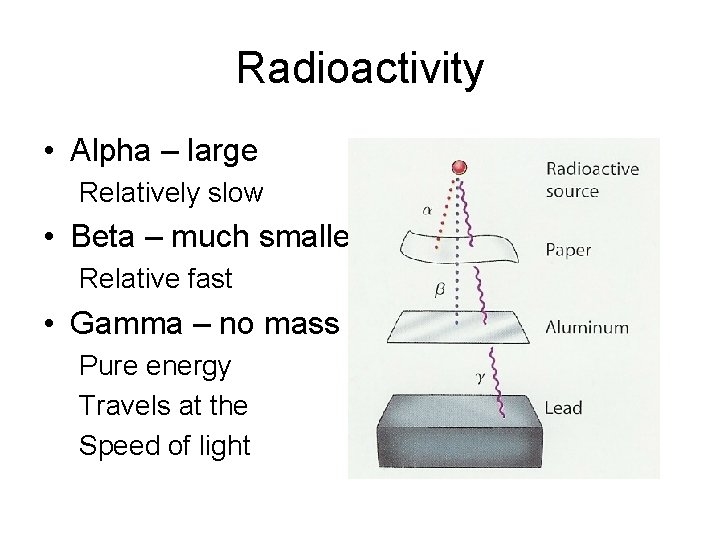
Radioactivity • Alpha – large Relatively slow • Beta – much smaller Relative fast • Gamma – no mass Pure energy Travels at the Speed of light

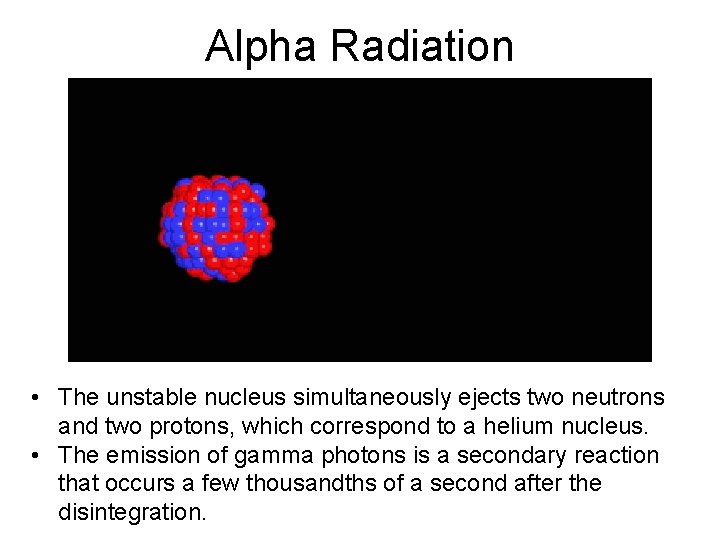
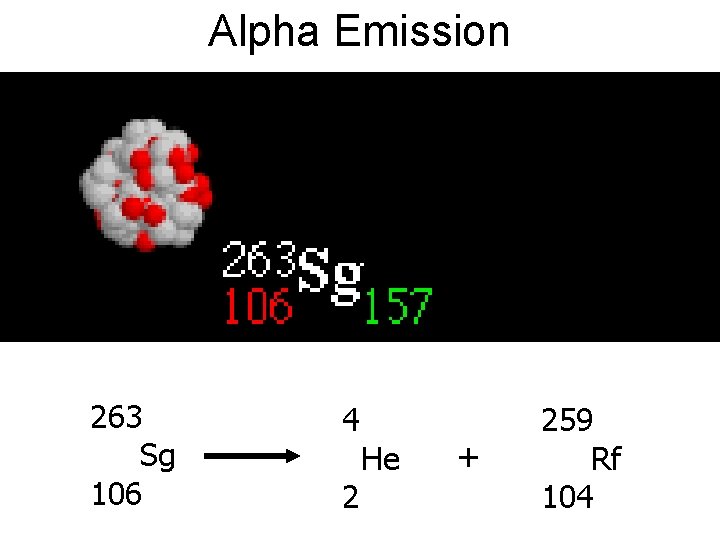
Alpha Radiation • The unstable nucleus simultaneously ejects two neutrons and two protons, which correspond to a helium nucleus. • The emission of gamma photons is a secondary reaction that occurs a few thousandths of a second after the disintegration.

Alpha Emission 263 Sg 106 4 2 He + 259 Rf 104

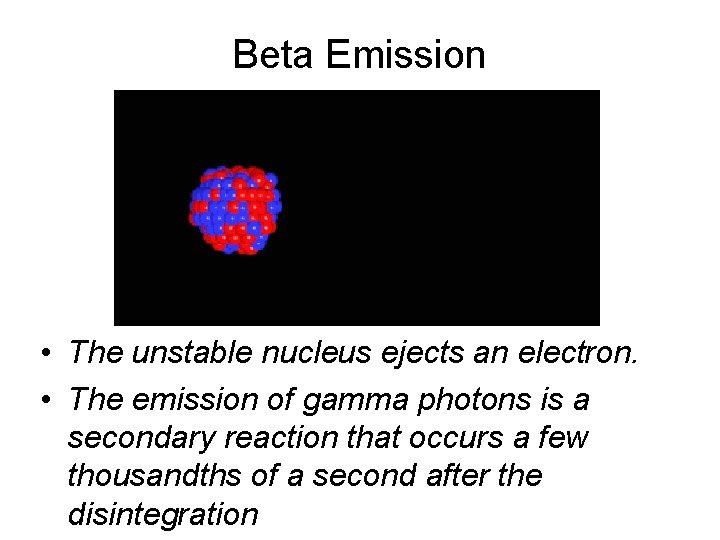
Beta Emission • The unstable nucleus ejects an electron. • The emission of gamma photons is a secondary reaction that occurs a few thousandths of a second after the disintegration

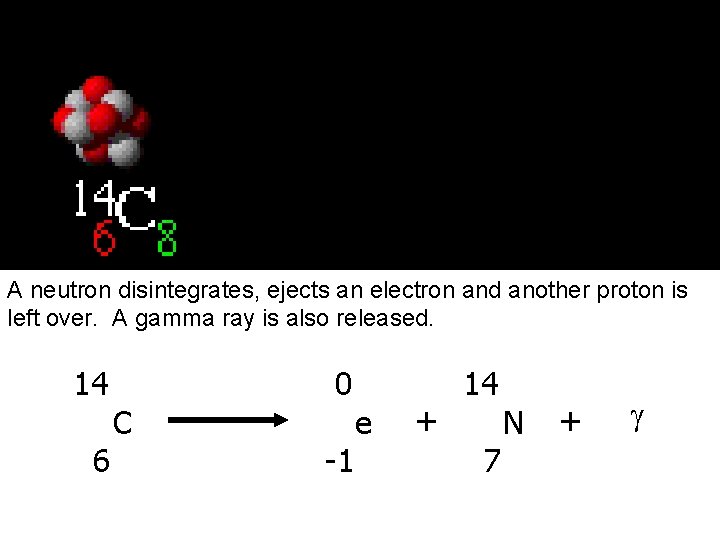
Beta Emission A neutron disintegrates, ejects an electron and another proton is left over. A gamma ray is also released. 14 6 C 0 -1 e + 14 7 N + g

Gamma Radiation involves high energy waves that don’t change the identity of the nucleus

Nuclear Reactions • Radioactivity results from changes in atomic nuclei. • Fission – splitting of a large nucleus into smaller pieces releases energy. • Fusion – small nuclei join to make a larger nucleus and release energy. • Energy is released when a small amount of mass converts to energy as E = mc 2. • The atomic masses of isotopes deviate from whole numbers due to loss of mass from energy released as they fused.

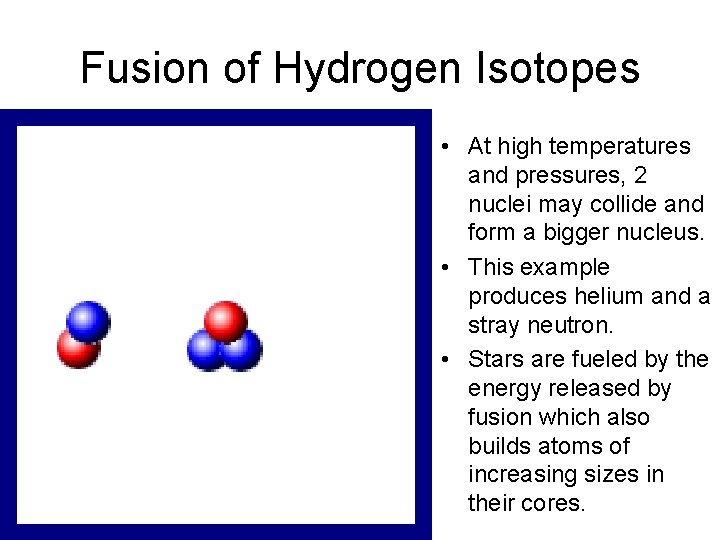
Fusion of Hydrogen Isotopes • At high temperatures and pressures, 2 nuclei may collide and form a bigger nucleus. • This example produces helium and a stray neutron. • Stars are fueled by the energy released by fusion which also builds atoms of increasing sizes in their cores.

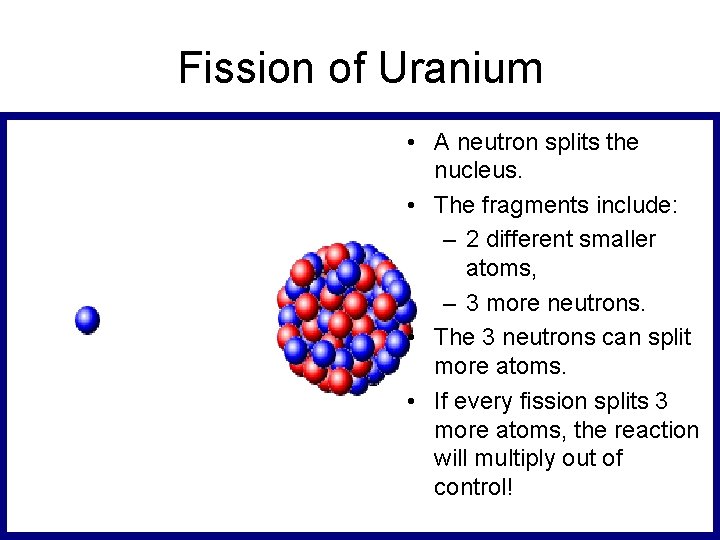
Fission of Uranium • A neutron splits the nucleus. • The fragments include: – 2 different smaller atoms, – 3 more neutrons. • The 3 neutrons can split more atoms. • If every fission splits 3 more atoms, the reaction will multiply out of control!

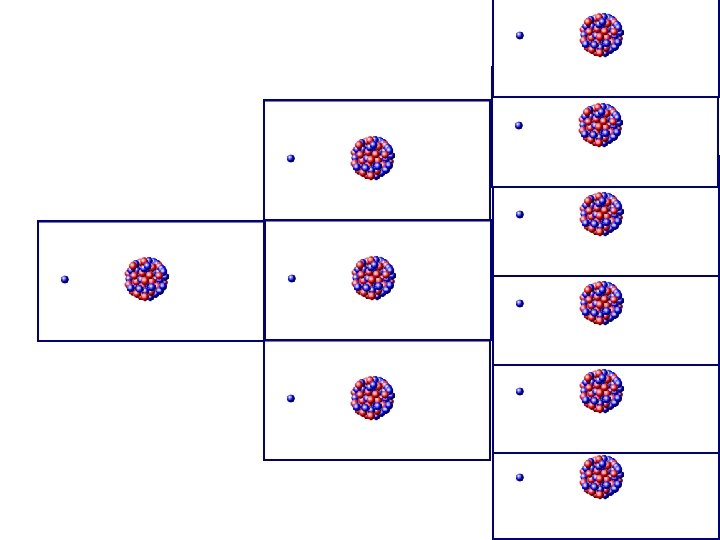
Nuclear Chain Reaction

Nuclear Equations • Styrofoam Demos • Alpha Decay – releases a helium nucleus. • Beta Decay – a neutron converts to a proton and releases an electron. • Assignment: Uranium Decay Series

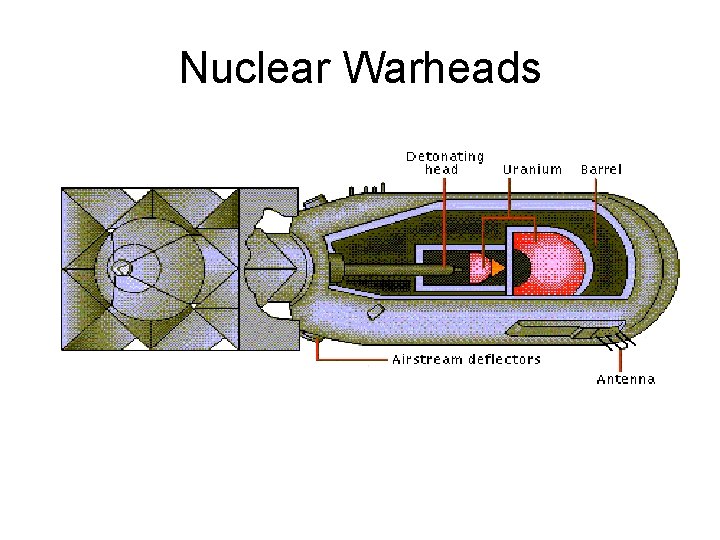
Nuclear Warheads

Chernobyl Nuclear Disaster

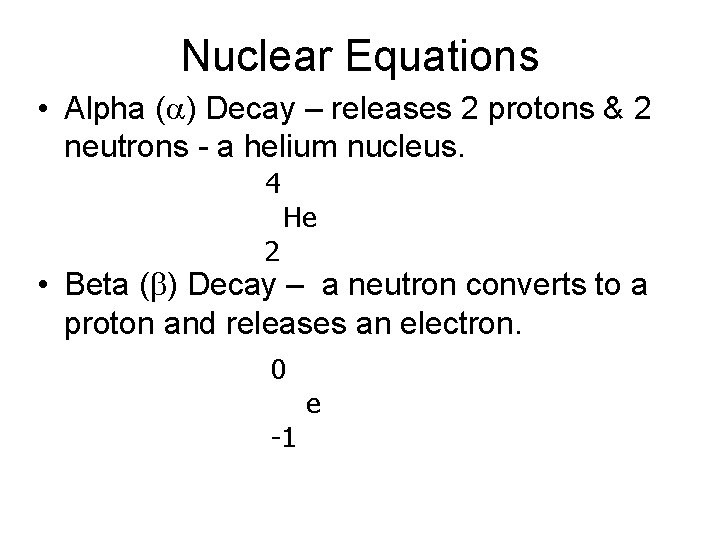
Nuclear Equations • Alpha (a) Decay – releases 2 protons & 2 neutrons - a helium nucleus. 4 2 He • Beta (b) Decay – a neutron converts to a proton and releases an electron. 0 -1 e

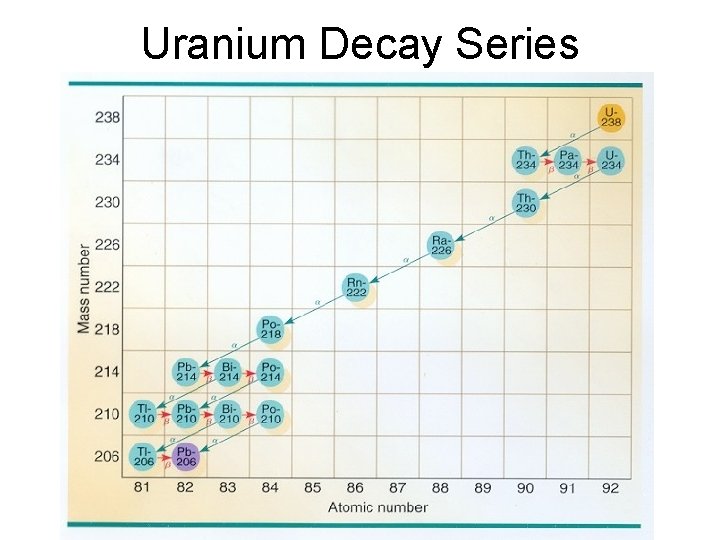
Uranium Decay Series

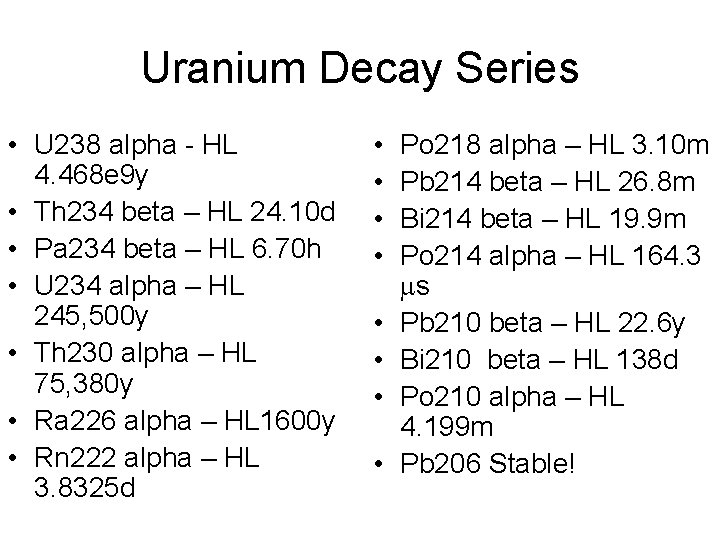
Uranium Decay Series • U 238 alpha - HL 4. 468 e 9 y • Th 234 beta – HL 24. 10 d • Pa 234 beta – HL 6. 70 h • U 234 alpha – HL 245, 500 y • Th 230 alpha – HL 75, 380 y • Ra 226 alpha – HL 1600 y • Rn 222 alpha – HL 3. 8325 d • • Po 218 alpha – HL 3. 10 m Pb 214 beta – HL 26. 8 m Bi 214 beta – HL 19. 9 m Po 214 alpha – HL 164. 3 ms Pb 210 beta – HL 22. 6 y Bi 210 beta – HL 138 d Po 210 alpha – HL 4. 199 m Pb 206 Stable!

Nuclear Equations • Uranium 238 does alpha decay: 238 234 a 4 U He + Th 92 2 90 – Mass numbers balance on both sides. – Atomic numbers balance on both sides • Thorium 234 then does beta decay: 234 b 0 Th e 90 -1 234 + Pa 91

Nuclear Equations Problems 1. 2. 3. 4. 5. U– 238 does alpha decay in nuclear reactors. Am-241 does alpha decay in smoke alarms. Tc-98 does beta decay in medical exams. C– 14 does beta decay in carbon dating. The Curies used Ra-226 which does alpha decay. 6. Co– 60 does beta decay in food irradiation. 7. Th-232 does alpha decay in camp lanterns. 8. P-35 does beta decay in DNA studies (Place isotopes activity in Outbox)

Nuclear Equations Quiz 1. Write the nuclear equation for the alpha decay of Iodine 131. 2. Write the nuclear equation for the beta decay of cobalt 60.

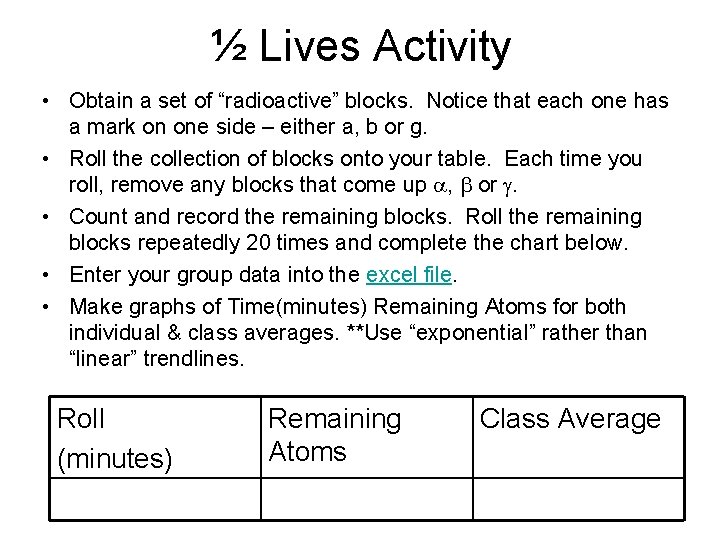
½ Lives Activity • Obtain a set of “radioactive” blocks. Notice that each one has a mark on one side – either a, b or g. • Roll the collection of blocks onto your table. Each time you roll, remove any blocks that come up a, b or g. • Count and record the remaining blocks. Roll the remaining blocks repeatedly 20 times and complete the chart below. • Enter your group data into the excel file. • Make graphs of Time(minutes) Remaining Atoms for both individual & class averages. **Use “exponential” rather than “linear” trendlines. Roll (minutes) Remaining Atoms Class Average
½ Lives Activity Questions 1. How do your lab pair results compare with the class average results? 2. Use the class average results and compute the 1 st ½ life, 2 nd ½ life, average ½ life. 3. What importance do ½ lives have to society? (dating, medical uses, wastes)

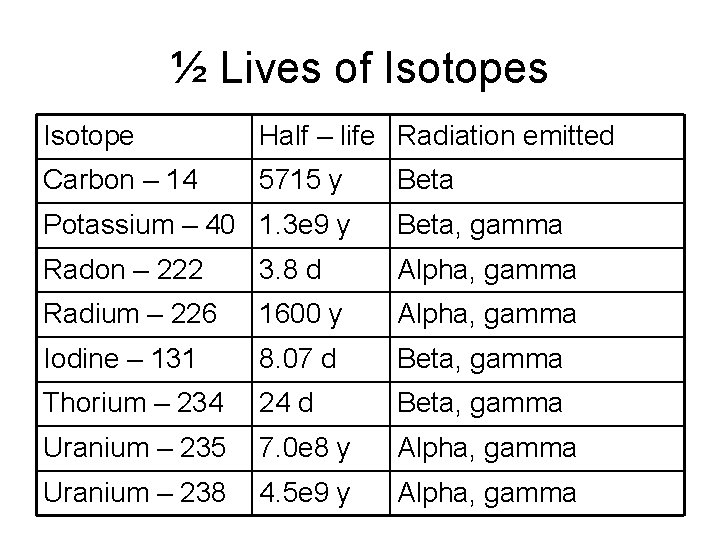
½ Lives • Each radio-isotope decays at a characteristic rate. • The decay rate is determined by the time that it takes for ½ of the radio-isotope nuclei to break down by fission. • Each ½ life reduces the remaining number of radioactive atoms by ½. • The number remaining approaches but never reaches zero. • Example: Iodine 131 has a ½ life of 8 days. How much of 1. 00 gram sample would remain after 24 days?

½ Lives of Isotopes Isotope Half – life Radiation emitted Carbon – 14 5715 y Beta Potassium – 40 1. 3 e 9 y Beta, gamma Radon – 222 3. 8 d Alpha, gamma Radium – 226 1600 y Alpha, gamma Iodine – 131 8. 07 d Beta, gamma Thorium – 234 24 d Beta, gamma Uranium – 235 7. 0 e 8 y Alpha, gamma Uranium – 238 4. 5 e 9 y Alpha, gamma

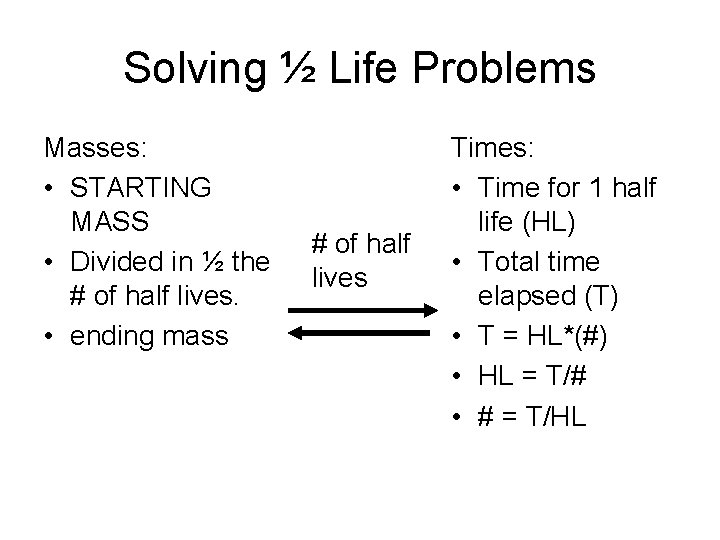
Solving ½ Life Problems Masses: • STARTING MASS • Divided in ½ the # of half lives. • ending mass # of half lives Times: • Time for 1 half life (HL) • Total time elapsed (T) • T = HL*(#) • HL = T/# • # = T/HL
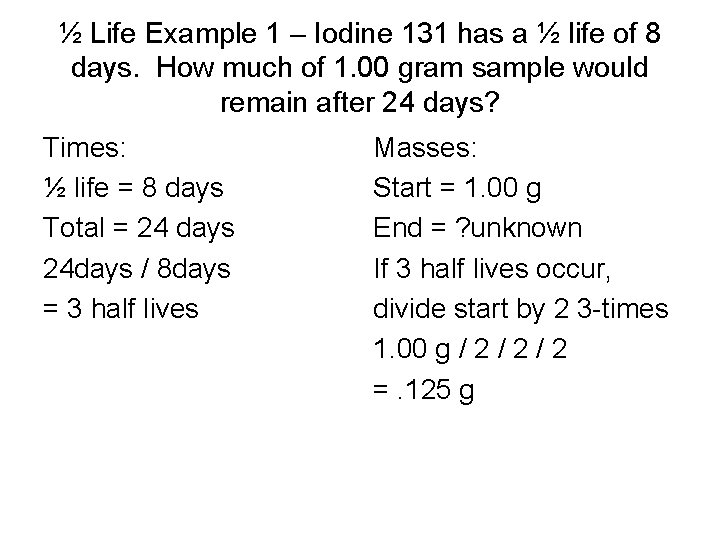
½ Life Example 1 – Iodine 131 has a ½ life of 8 days. How much of 1. 00 gram sample would remain after 24 days? Times: ½ life = 8 days Total = 24 days 24 days / 8 days = 3 half lives Masses: Start = 1. 00 g End = ? unknown If 3 half lives occur, divide start by 2 3 -times 1. 00 g / 2 / 2 =. 125 g

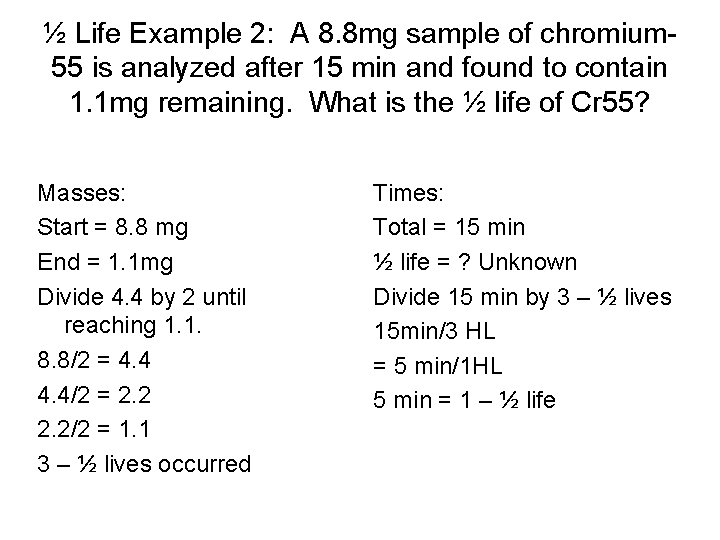
½ Life Example 2: A 8. 8 mg sample of chromium 55 is analyzed after 15 min and found to contain 1. 1 mg remaining. What is the ½ life of Cr 55? Masses: Start = 8. 8 mg End = 1. 1 mg Divide 4. 4 by 2 until reaching 1. 1. 8. 8/2 = 4. 4/2 = 2. 2/2 = 1. 1 3 – ½ lives occurred Times: Total = 15 min ½ life = ? Unknown Divide 15 min by 3 – ½ lives 15 min/3 HL = 5 min/1 HL 5 min = 1 – ½ life

½ Life Problems 1. If you have $1 million dollars and every 2 seconds it decreases by 1/2, how long will it take until you are penniless? 2. If a sample of a fossil mammoth has 1/8 th the amount of carbon 14 as it would today, how old must the fossil be? (1/2 L C 14 = 5715 years. 3. If a rock contained 1. 2 g of potassium 40 when it formed, how many grams remain after 4 billion years. (1/2 L K 40 = 1. 3 e 9 y) Asmt: P 780 #1&2, P 803 #24&25

More ½ Life Problems 4. If a sample of radioactive isotope has a half-life of 1 year, how much of the original sample will be left at the end of the second year? The third year? The fourth year? 5. The isotope cesium-137, which has a half-life of 30 years, is a product of nuclear power plants. How long will it take for this isotope to decay to about one-sixteenth its original amount? 6. Iodine-131 has a half-life of 8 days. What fraction of the original sample would remain at the end of 32 days? 7. The half-life of chromium-51 is 28 days. If the sample contained 510 grams, how much chromium would remain after 56 days? How much would remain after 1 year?

½ Lives Quiz 1. A sample of a radioactive isotope with an original mass of 8. 00 g is observed for 30 days. After that time, 0. 25 g of the isotope remains. What is its half-life? 2. The starting mass of a radioactive isotope is 20. 0 g. The half-life period of this isotope is 2 days. The sample is observed for 14 days. What PERCENTAGE of the original amount remains after 14 days?

Health Physics Society • http: //hps. org/publicinformation/ate/q 754. html • Q: What are some health effects of the element uranium? • A: The toxicity of uranium has been under study for over 50 years, including life-span studies in small animals. Depleted uranium and natural uranium both consist primarily of the uranium isotope 238 U. They are only very weakly radioactive and are not hazardous radioactive toxicants, but uranium is a weak chemical poison that can seriously damage the kidneys at high blood concentrations. Virtually all of the observed or expected effects are from nephrotoxicity associated with deposition in the kidney tubules and glomeruli damage at high blood concentrations of uranium. The ionizing radiation doses from depleted and natural uranium are very small compared to potential toxic effects from uranium ions in the body (primarily damage to kidney tubules).