Atomic Models and Subatomic Particles The ancient Greeks
Atomic Models and Subatomic Particles

The ancient Greeks first proposed that all matter is made up of particles more than 2000 years ago.

However, they thought all matter was composed of only 4 elements: earth, wind, fire and water!

In the 1600’s, Robert Boyle identified gold and silver as being elements. Other elements were soon discovered.

The Greek concept of only 4 elements (earth, wind, fire, and water) was no longer accepted!

In the 1700’s, John Dalton proposed that all matter is composed of tiny particles called atoms.

Dalton thought all atoms of the same element were identical.
Atoms of different elements were different because they had different masses.
Compounds were formed by different combinations of different elements.
The “Cannonball” Model

In the late 1800’s, J. J. Thomson discovered electrons. He then proposed atoms consisted of both positive and negative charges.
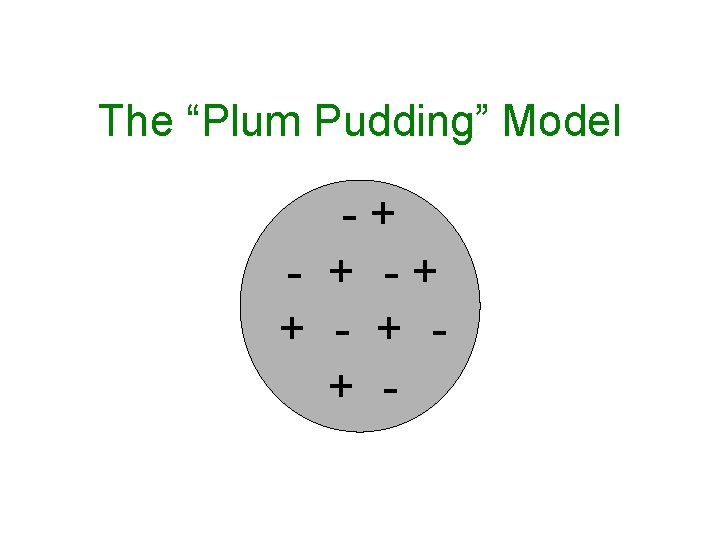
The “Plum Pudding” Model -+ - + -+ + -

In the early 1900’s, Ernest Rutherford carried out his very famous “Gold Foil” experiment.
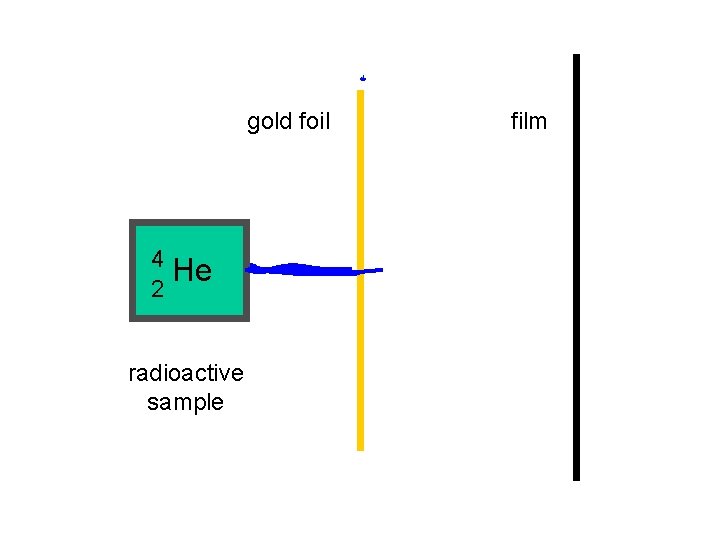
gold foil 4 He 2 radioactive sample film
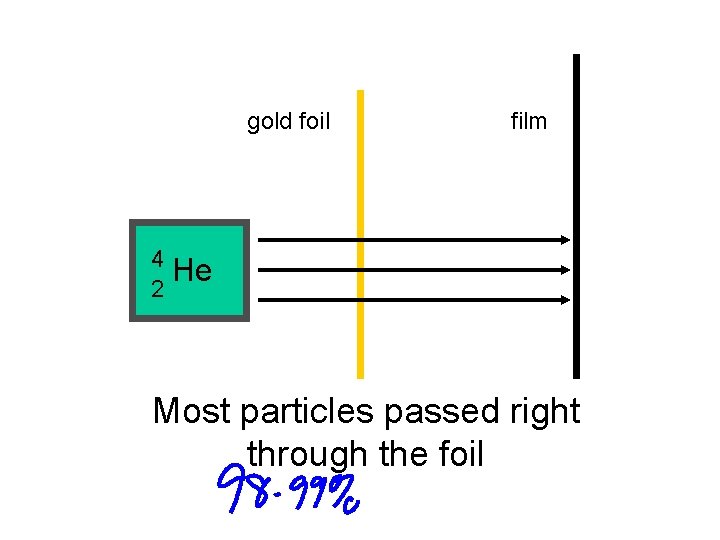
gold foil film 4 He 2 Most particles passed right through the foil
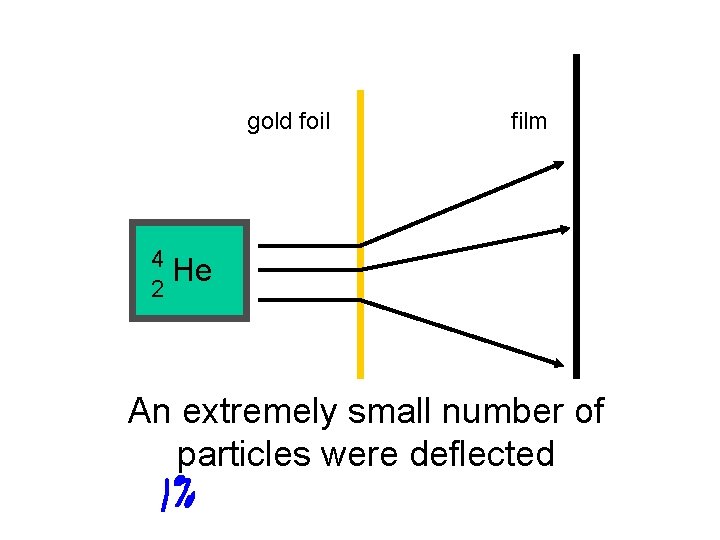
gold foil film 4 He 2 An extremely small number of particles were deflected
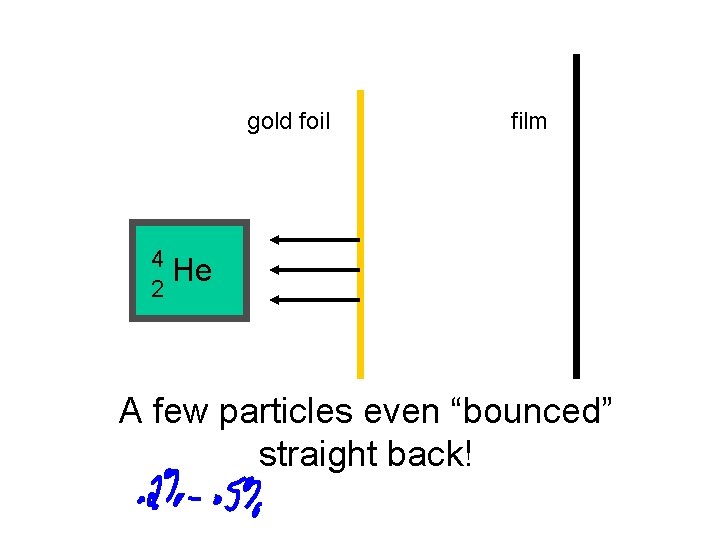
gold foil film 4 He 2 A few particles even “bounced” straight back!

Ernest Rutherford’s Gold Foil Experiment provided 2 extremely important conclusions!

1. An atom must consist of mainly empty space 2. There must be a very tiny central region (nucleus) with a positive charge
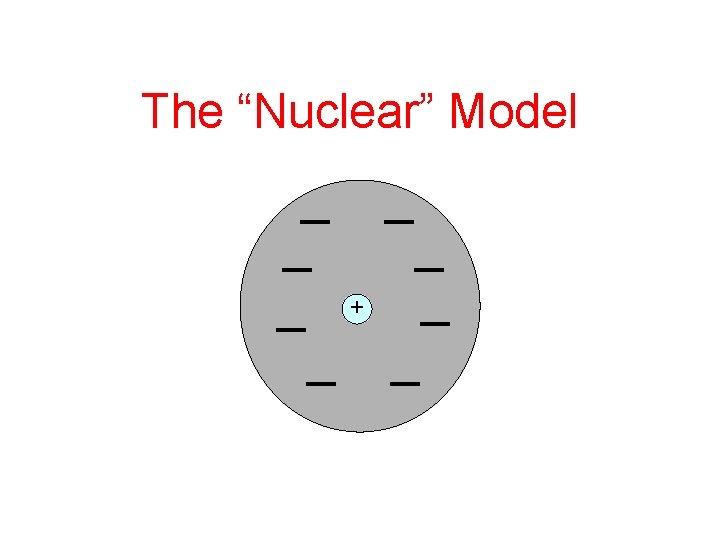
The “Nuclear” Model +
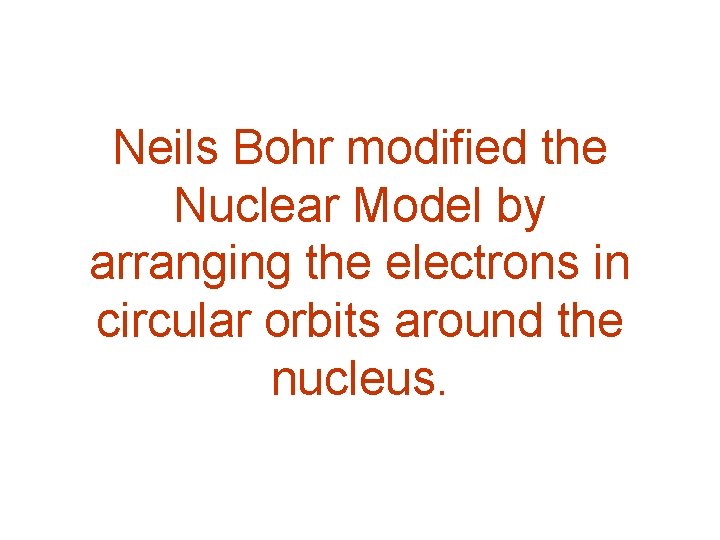
Neils Bohr modified the Nuclear Model by arranging the electrons in circular orbits around the nucleus.
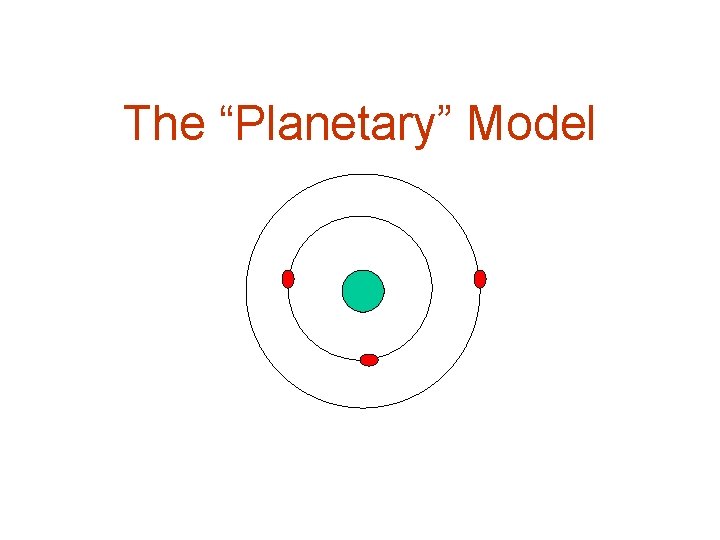
The “Planetary” Model

The current model of the atom is called the “Orbital” or “Wave-Mechanical” Model.

All atoms are made up of smaller particles called “subatomic particles”. You need to know the 3 most important ones!

A proton has a charge of 1+ and an average mass of about 1 u. Protons are found in the nucleus of an atom.

A neutron has no charge (is neutral) and also has an average mass of about 1 u. Neutrons are also found in the nucleus.
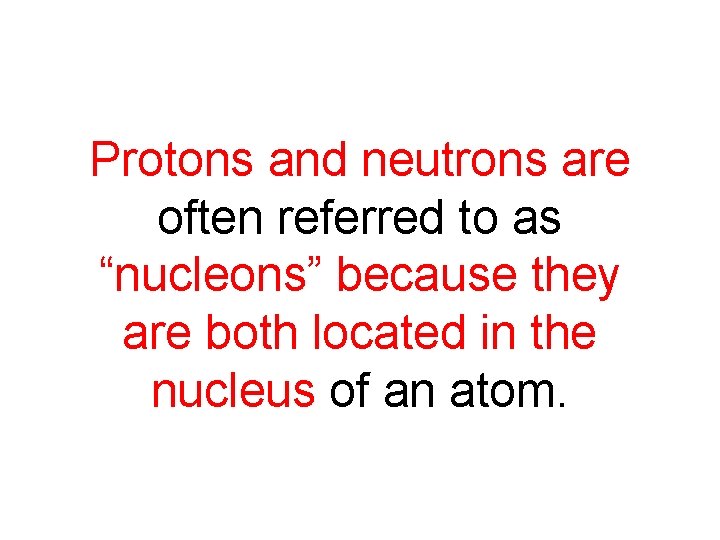
Protons and neutrons are often referred to as “nucleons” because they are both located in the nucleus of an atom.

An electron has a charge of 1 -. Electrons are found in various regions outside of the nucleus.

It takes 1836 electrons to equal the mass of 1 proton or neutron. For our purposes, we say an electron has a mass of O.
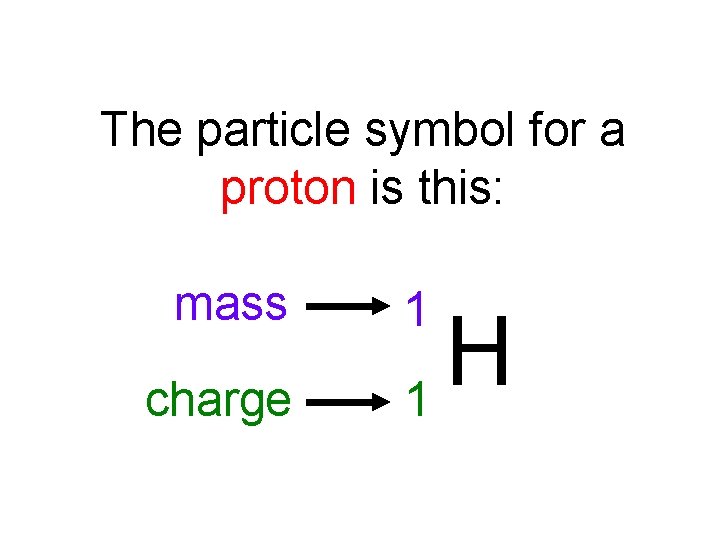
The particle symbol for a proton is this: mass charge 1 H 1
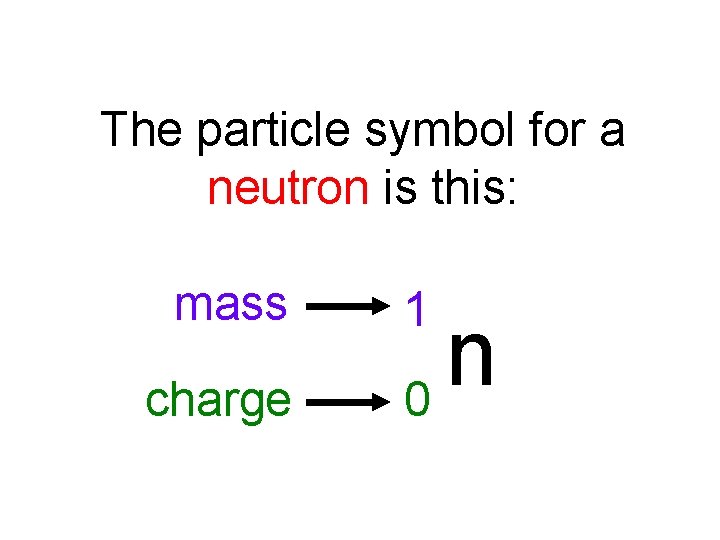
The particle symbol for a neutron is this: mass charge 1 n 0
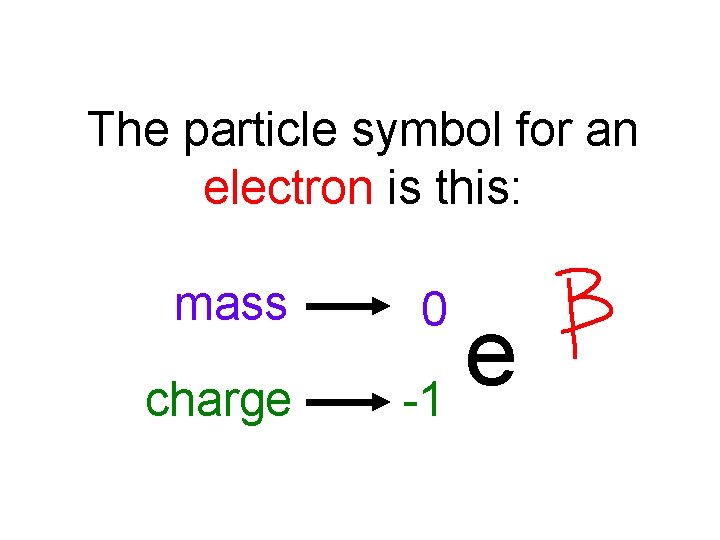
The particle symbol for an electron is this: mass charge 0 e -1

I you can’t remember all this, you don’t have to. Just remember to look at Table O!
Elements are different because they contain different numbers of protons, neutrons, and electrons.
The number of protons determines what the element is. Atoms of the same element must have the same number of protons!

The “Atomic Number” (charge) is the number of protons in atoms of that element.
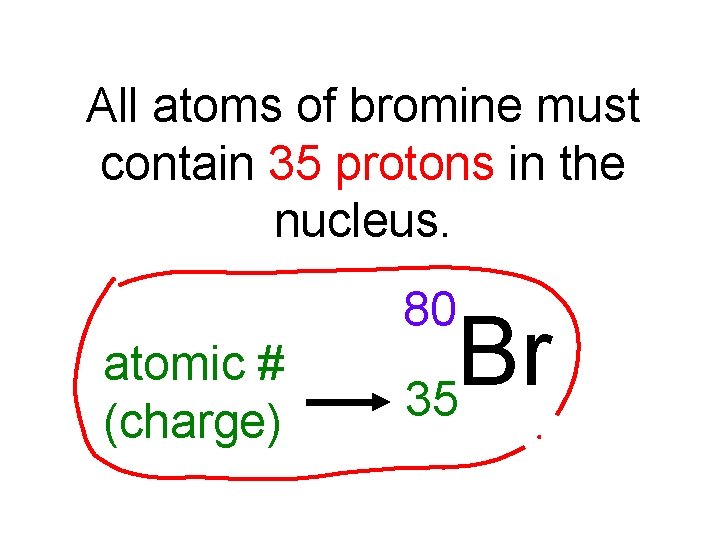
All atoms of bromine must contain 35 protons in the nucleus. 80 atomic # (charge) Br 35
By definition, an atom is neutral. This means the number of protons (+ charges) and electrons (- charges) must be the same.

However, atoms can lose or gain electrons when they react chemically. When they do, they become charged particles called “ions”.
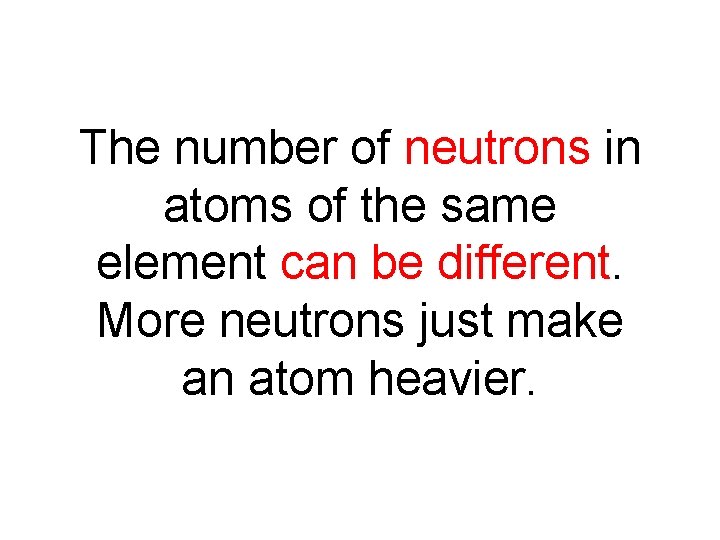
The number of neutrons in atoms of the same element can be different. More neutrons just make an atom heavier.
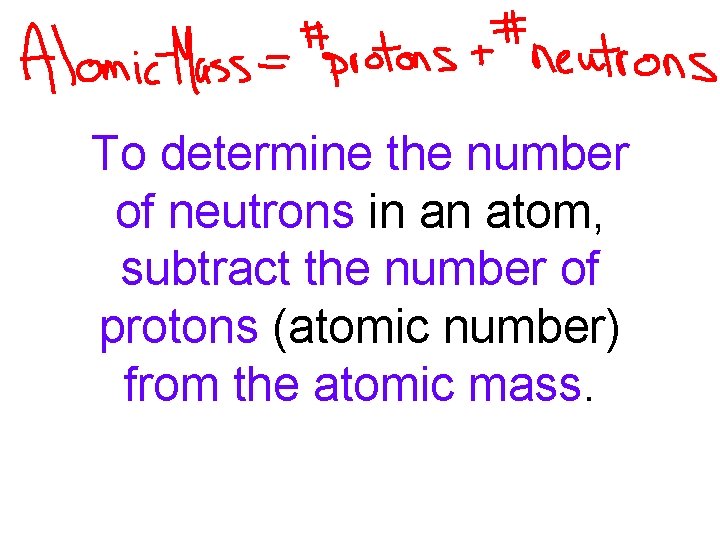
To determine the number of neutrons in an atom, subtract the number of protons (atomic number) from the atomic mass.
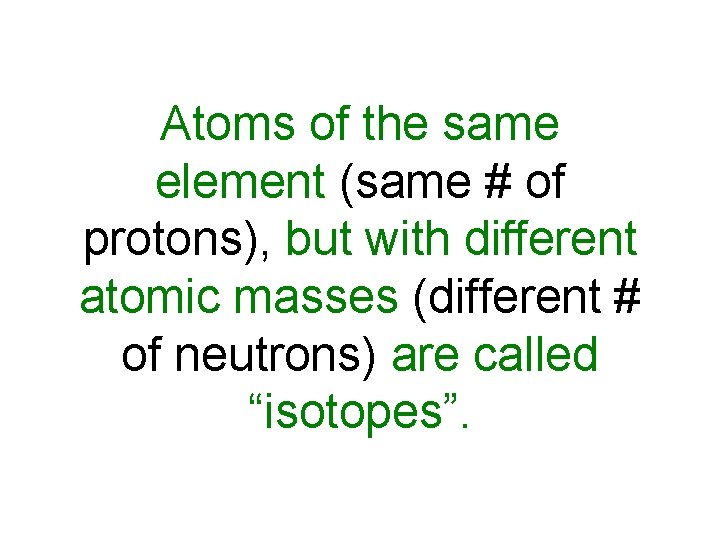
Atoms of the same element (same # of protons), but with different atomic masses (different # of neutrons) are called “isotopes”.
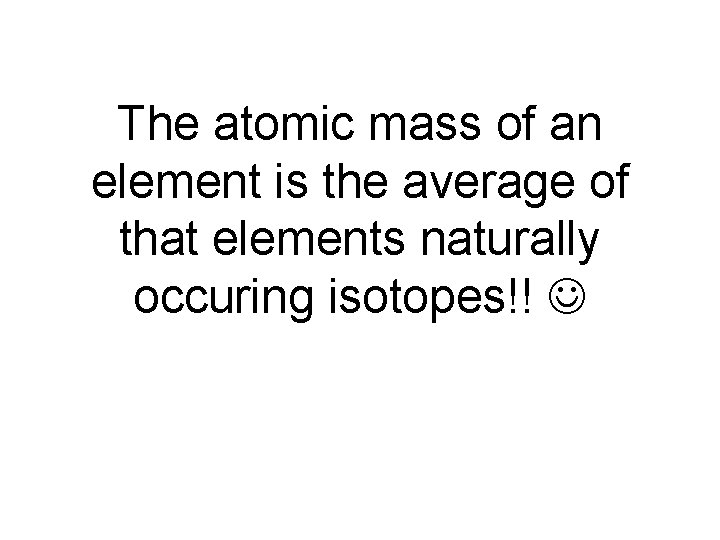
The atomic mass of an element is the average of that elements naturally occuring isotopes!!
- Slides: 43