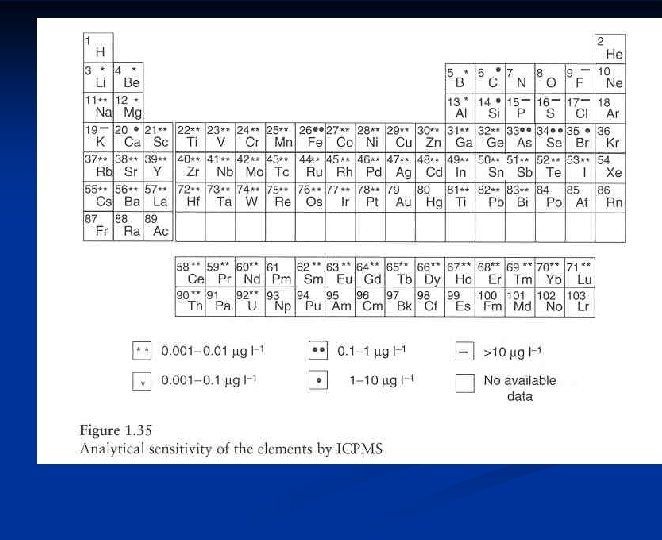
Atomic Mass Spectrometry Nearly all elements in the




















- Slides: 45

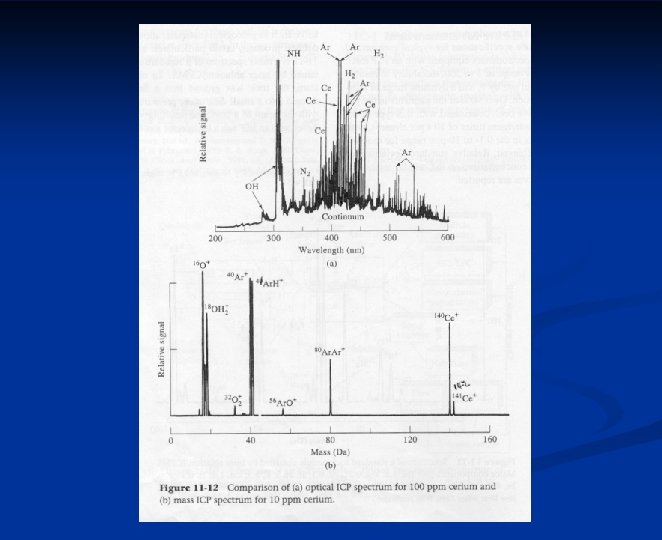
Atomic Mass Spectrometry Nearly all elements in the periodic table can be determined by mass spectrometry n More selective and sensitive than optical instruments n Simple spectra n Isotope ratios n Much more expensive instrumentation n



Lets talk about mass! n Atomic mass of Carbon n Atomic mass of Chlorine n Atomic mass of Hydrogen

Lets talk about mass! n Atomic mass of Carbon n n Atomic mass of Chlorine n n 12. 00000000000000 amu 35. 4527 amu Atomic mass of Hydrogen n 1. 00794 amu 1 amu = 1 dalton (Da) Happy?

What about isotopes? n Atomic mass of Carbon n n Atomic mass of Chlorine n n 12. 000 amu for 12 C but 13. 3355 for 13 C 34. 9688 amu for 35 Cl and 36. 9659 for 37 Cl Atomic mass of Hydrogen n 1. 00794 amu for H and 2. 0141 for D! Get it now?

Just for clarification n n n Atomic mass amu, atomic mass units (uma? ? ) “Da” or Dalton. k. D (kilo. Dalton for macromolecules) 1 amu = 1. 66056*10 -27 kg. proton, mp = 1. 67265*10 -27 kg, neutron, mn = 1. 67495*10 -27 kg.

Exact mass and Nominal mass The nominal mass of a compound/ion is calculated by simply adding the integer masses of the isotopes of all elements contributing to the molecule n nominal mass of 12 C 1 H 379 Br n 12 C, 1 H, and 79 Br to obtain Mnominal= 94 amu. Chemical (Average) atomic weight n Consider each isotope and its fraction abundance in nature n Mass-to-charge ratio

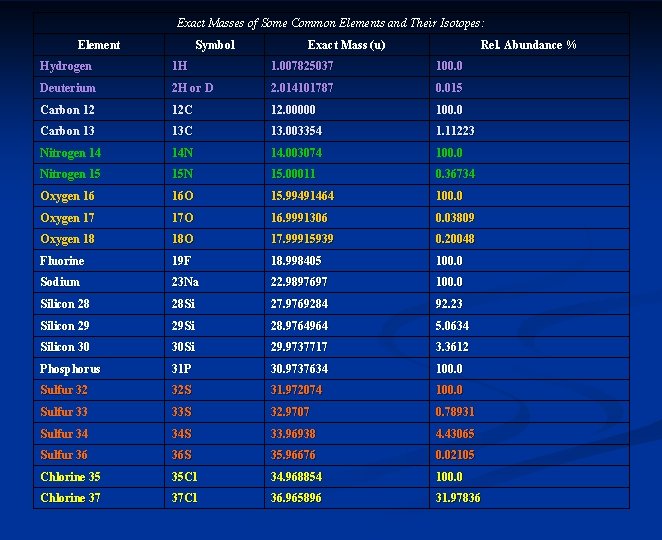
Exact Masses of Some Common Elements and Their Isotopes: Element Symbol Exact Mass (u) Rel. Abundance % Hydrogen 1 H 1. 007825037 100. 0 Deuterium 2 H or D 2. 014101787 0. 015 Carbon 12 12 C 12. 00000 100. 0 Carbon 13 13 C 13. 003354 1. 11223 Nitrogen 14 14 N 14. 003074 100. 0 Nitrogen 15 15 N 15. 00011 0. 36734 Oxygen 16 16 O 15. 99491464 100. 0 Oxygen 17 17 O 16. 9991306 0. 03809 Oxygen 18 18 O 17. 99915939 0. 20048 Fluorine 19 F 18. 998405 100. 0 Sodium 23 Na 22. 9897697 100. 0 Silicon 28 28 Si 27. 9769284 92. 23 Silicon 29 29 Si 28. 9764964 5. 0634 Silicon 30 30 Si 29. 9737717 3. 3612 Phosphorus 31 P 30. 9737634 100. 0 Sulfur 32 32 S 31. 972074 100. 0 Sulfur 33 33 S 32. 9707 0. 78931 Sulfur 34 34 S 33. 96938 4. 43065 Sulfur 36 36 S 35. 96676 0. 02105 Chlorine 35 35 Cl 34. 968854 100. 0 Chlorine 37 37 Cl 36. 965896 31. 97836

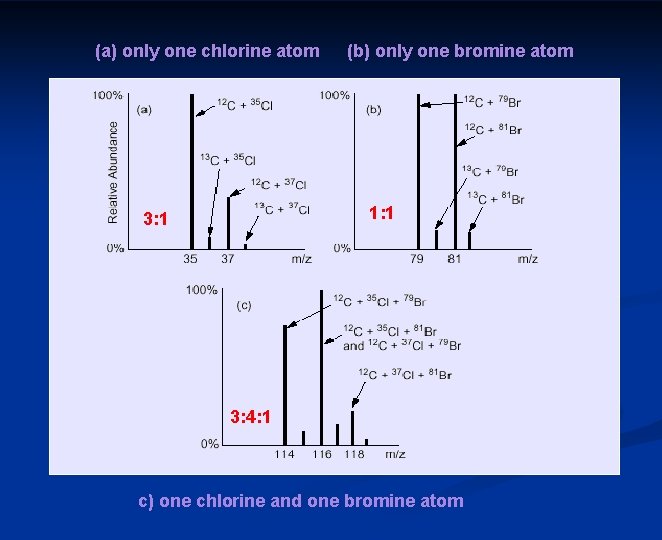
(a) only one chlorine atom (b) only one bromine atom 1: 1 3: 4: 1 c) one chlorine and one bromine atom

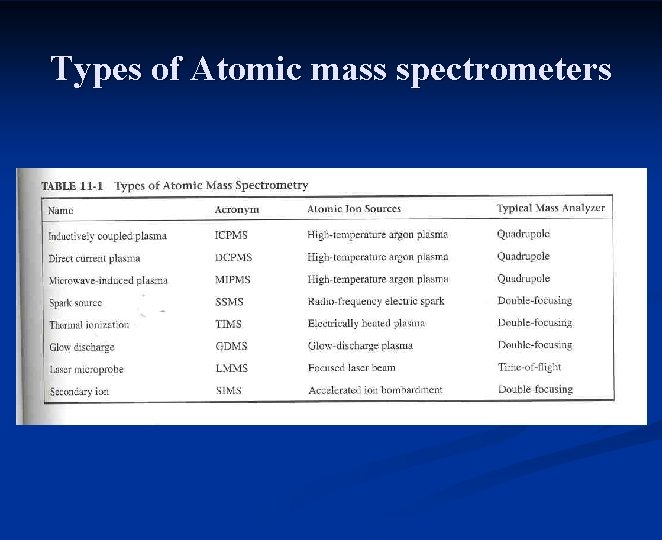
Types of Atomic mass spectrometers

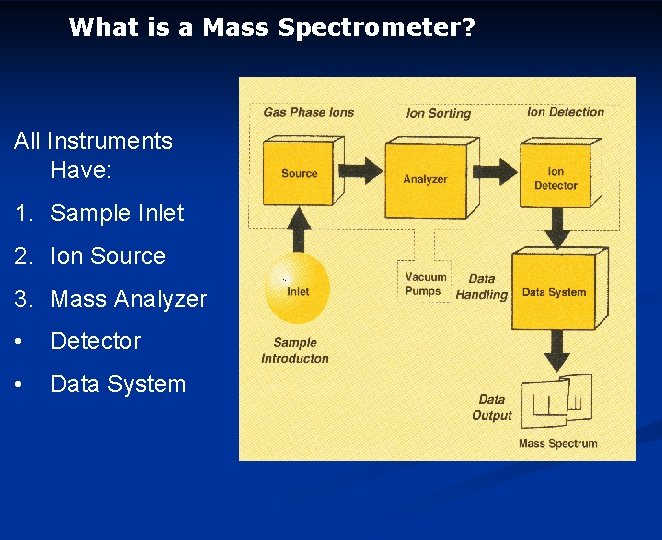
What is a Mass Spectrometer? All Instruments Have: 1. Sample Inlet 2. Ion Source 3. Mass Analyzer • Detector • Data System

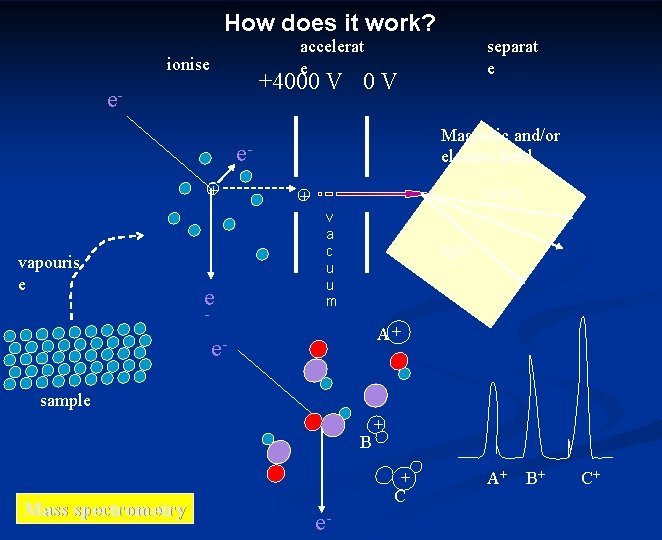
How does it work? accelerat e ionise separat e +4000 V e- Magnetic and/or electric field e+ vapouris e e - heavy + v a c u u m light A+ esample B + + Mass spectrometry C e- A+ B+ C+

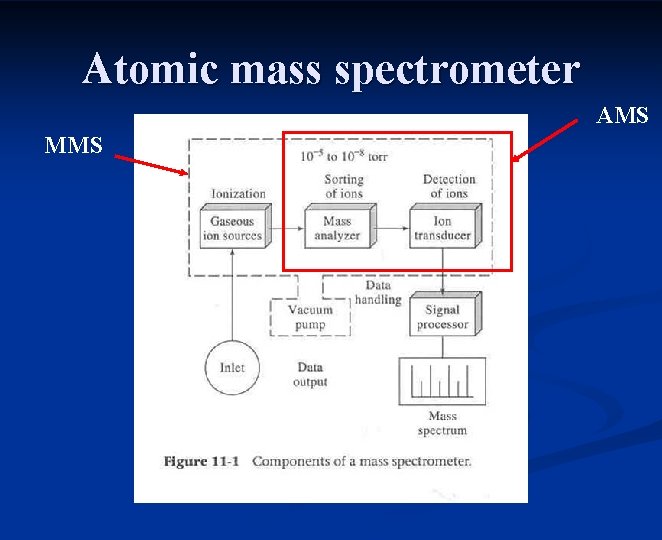
Atomic mass spectrometer AMS MMS

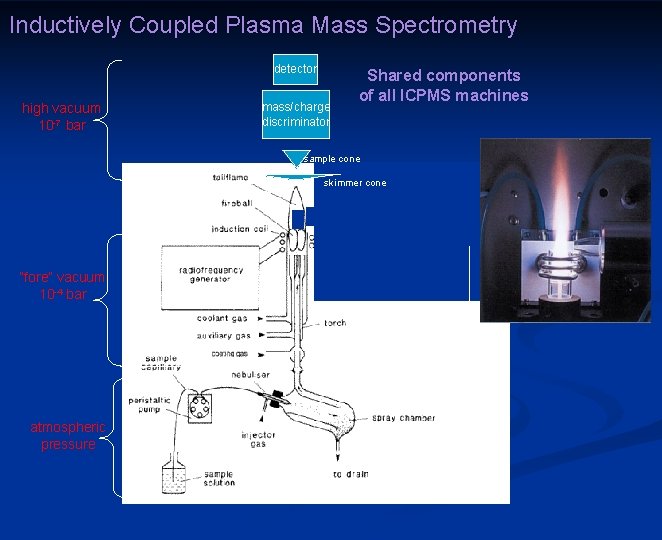
Inductively Coupled Plasma Mass Spectrometry detector high vacuum 10 -7 bar mass/charge discriminator Shared components of all ICPMS machines sample cone skimmer cone “fore” vacuum 10 -4 bar instrument housing atmospheric pressure

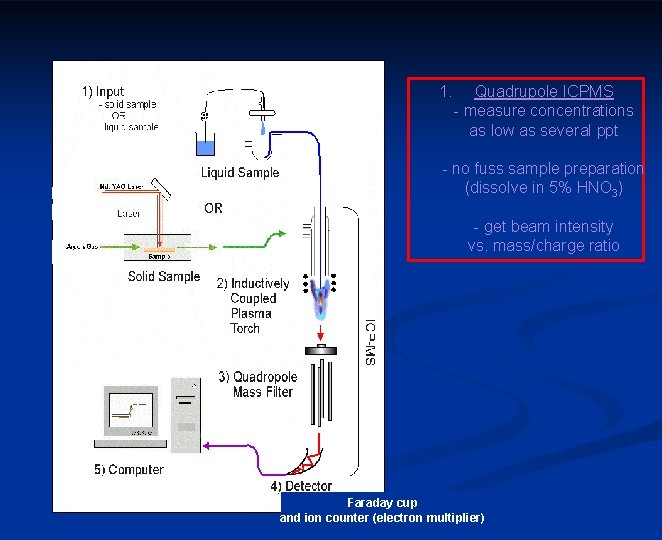
1. Quadrupole ICPMS - measure concentrations as low as several ppt - no fuss sample preparation (dissolve in 5% HNO 3) - get beam intensity vs. mass/charge ratio or magnetic sector Faraday cup and ion counter (electron multiplier)

AGILENT 7500 OMEGA LENS

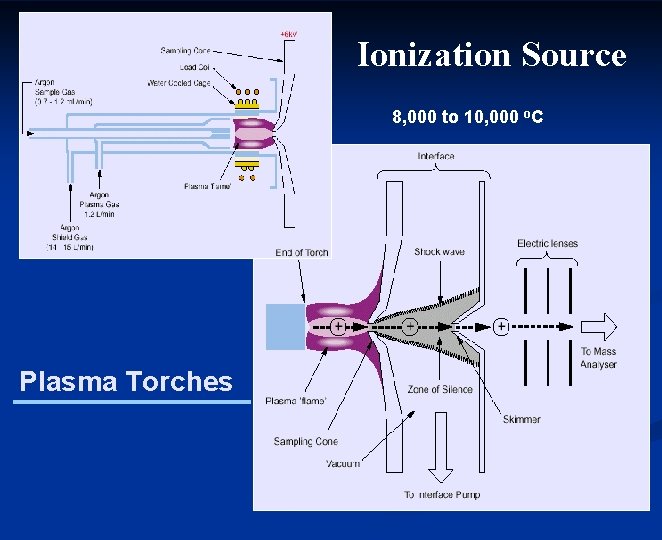
Ionization Source 8, 000 to 10, 000 o. C Plasma Torches

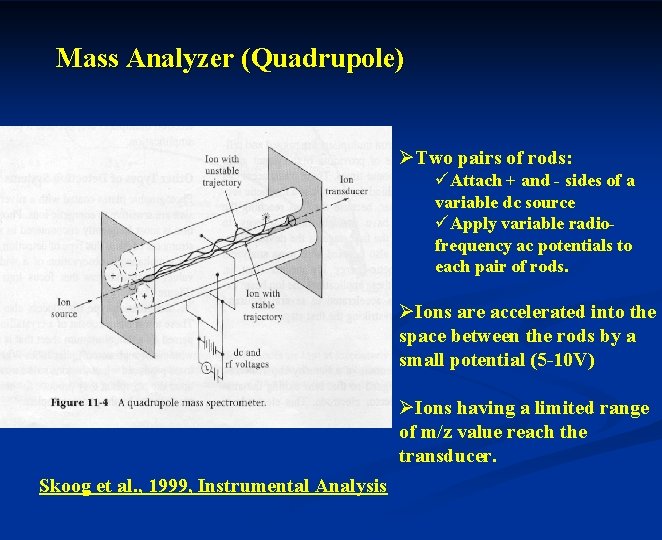
Mass Analyzer (Quadrupole) ØTwo pairs of rods: üAttach + and - sides of a variable dc source üApply variable radiofrequency ac potentials to each pair of rods. ØIons are accelerated into the space between the rods by a small potential (5 -10 V) ØIons having a limited range of m/z value reach the transducer. Skoog et al. , 1999, Instrumental Analysis

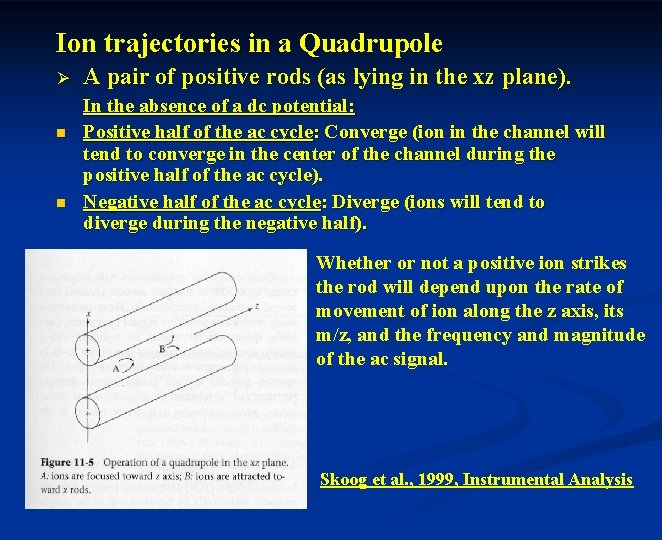
Ion trajectories in a Quadrupole Ø n n A pair of positive rods (as lying in the xz plane). In the absence of a dc potential: Positive half of the ac cycle: Converge (ion in the channel will tend to converge in the center of the channel during the positive half of the ac cycle). Negative half of the ac cycle: Diverge (ions will tend to diverge during the negative half). Whether or not a positive ion strikes the rod will depend upon the rate of movement of ion along the z axis, its m/z, and the frequency and magnitude of the ac signal. Skoog et al. , 1999, Instrumental Analysis

Ø A pair of positive rods (Cont’d) With dc potential: Heavier ions: less affected by ac (largely by dc). Lighter ions: deflected during negative cycle of ac. The pair of positive rods: a high-pass mass filter for positive ions traveling in the xz plane.

Ø The pair of negative rods In the absence of the ac potential: All positive ions will tend to strike the rods. With ac potential: For the lighter ions, however, this movement may be offset by the positive half cycle of ac potential. Thus, the pair of negative rods operates as a low-pass mass filter. The mass that can be analyzed can be varied by adjusting the ac and dc potential.

How does it work?


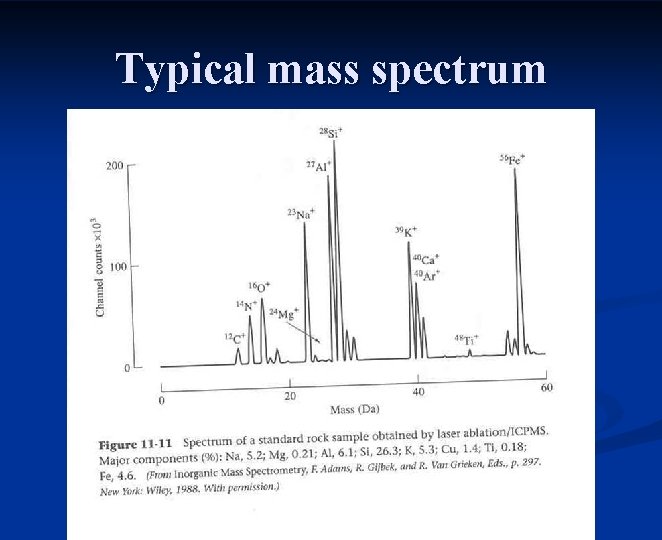
Typical mass spectrum

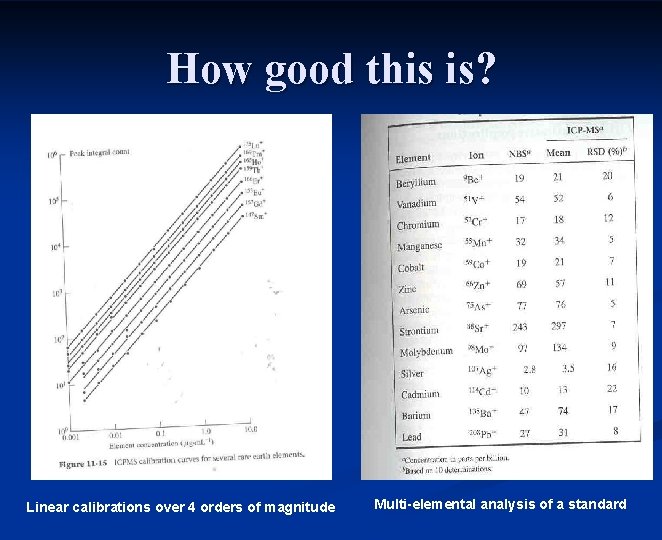
How good this is? Linear calibrations over 4 orders of magnitude Multi-elemental analysis of a standard

ICP-MS: a handy tool!

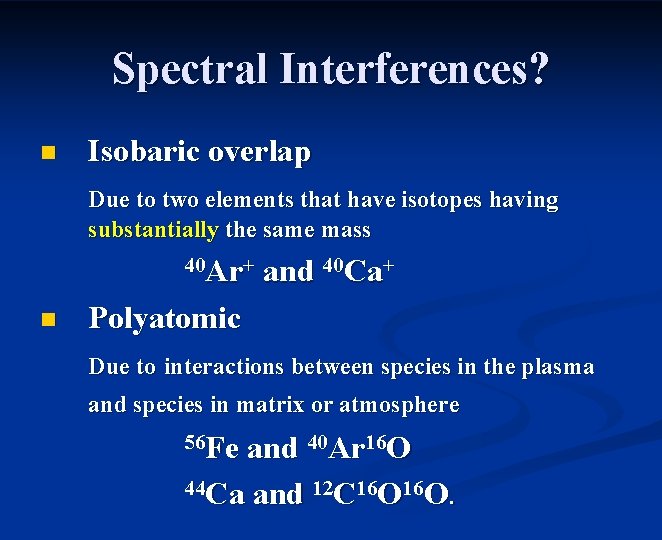
Spectral Interferences? n Isobaric overlap Due to two elements that have isotopes having substantially the same mass 40 Ar+ n and 40 Ca+ Polyatomic Due to interactions between species in the plasma and species in matrix or atmosphere 56 Fe and 40 Ar 16 O 44 Ca and 12 C 16 O.

Isobaric interferences?

Spectral Interferences? n Refractory oxide As a result of incomplete dissociation of the sample matrix or from recombination in the plasma tail MO+, MO 2+, MO 3+ n Doubly charged ions

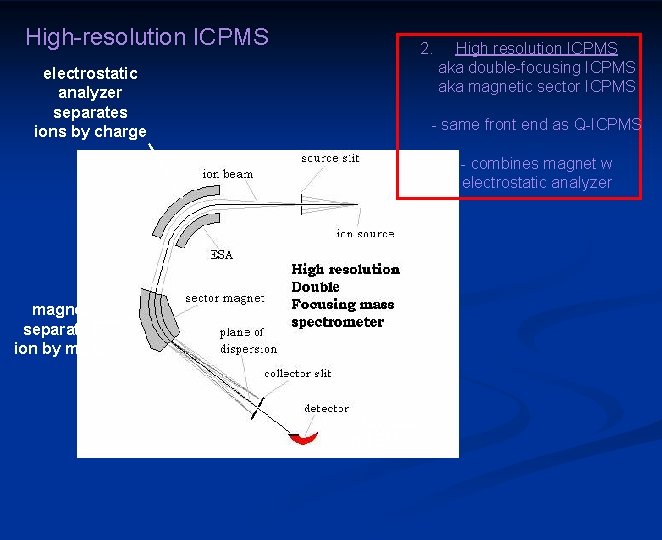
High-resolution ICPMS 2. electrostatic analyzer separates ions by charge High resolution ICPMS aka double-focusing ICPMS aka magnetic sector ICPMS - same front end as Q-ICPMS - combines magnet w electrostatic analyzer magnet separates ion by mass Faraday cup and EM

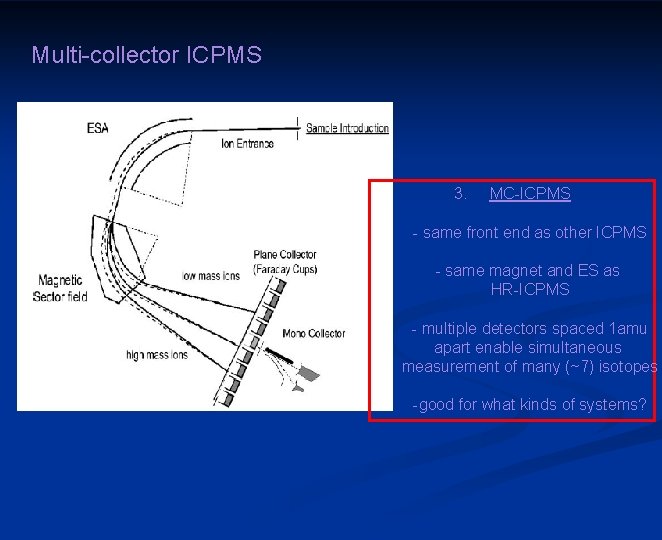
Multi-collector ICPMS 3. MC-ICPMS - same front end as other ICPMS - same magnet and ES as HR-ICPMS - multiple detectors spaced 1 amu apart enable simultaneous measurement of many (~7) isotopes -good for what kinds of systems?

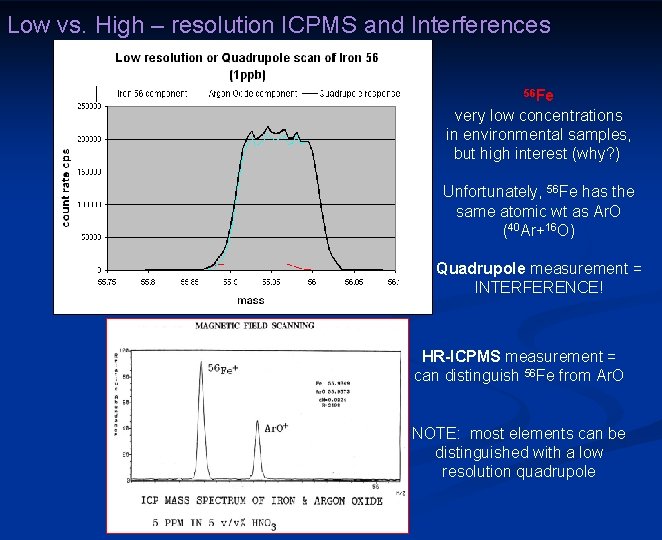
Low vs. High – resolution ICPMS and Interferences 56 Fe very low concentrations in environmental samples, but high interest (why? ) Unfortunately, 56 Fe has the same atomic wt as Ar. O (40 Ar+16 O) Quadrupole measurement = INTERFERENCE! HR-ICPMS measurement = can distinguish 56 Fe from Ar. O NOTE: most elements can be distinguished with a low resolution quadrupole

REMEMBER: all mass spectrometers are “black boxes” we really have no idea what goes on from sample container to detector signal Ex: you measure a count-rate of 10, 000 cps for a given element, but you need to know how many atoms of that element, or its concentration, were in your sample - measuring isotope ratios is a powerful approach because we can measure samples against standards with known isotopic ratios (it’s much more difficult to change a material’s isotopic ratios than it is to change its elemental concentration!) - isotope dilution takes advantage of ability to precisely measure ratios - ALL measurements need to include blanks and standards (either concentration or ratio standards)

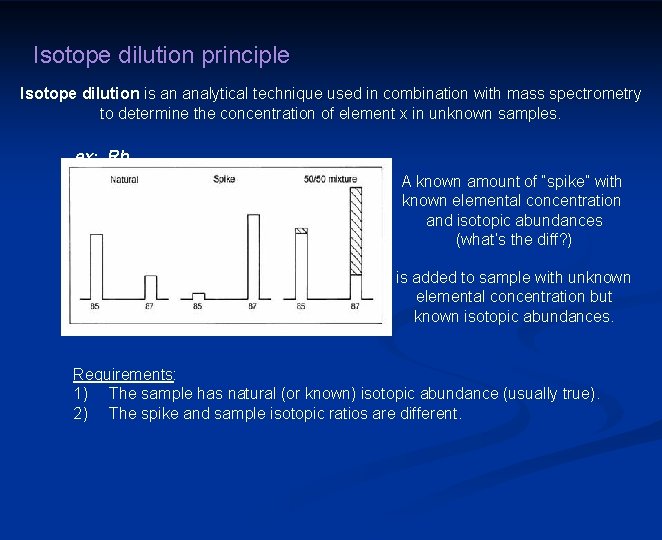
Isotope dilution principle Isotope dilution is an analytical technique used in combination with mass spectrometry to determine the concentration of element x in unknown samples. ex: Rb A known amount of “spike” with known elemental concentration and isotopic abundances (what’s the diff? ) is added to sample with unknown elemental concentration but known isotopic abundances. Requirements: 1) The sample has natural (or known) isotopic abundance (usually true). 2) The spike and sample isotopic ratios are different.

Advanced Analytical Chemistry – CHM 6157 University Updated on 9/13/2006 ® Y. CAI Florida International Chapter 3 ICPMS Isotope Dilution ü ü Isotope dilution is a super internal standard addition method on the basis of isotope ratios. Add a known amount (spike) of a stable enriched isotope of the element considered, which has at least two stable isotopes 1 and 2, to the sample Measure the isotope ratio of isotopes 1 and 2 in the Spike, the unspiked sample and finally the spiked sample. The concentration of the element of interest can then be deducted from these isotopic ratios and from the amount of spike added.

Advanced Analytical Chemistry – CHM 6157 University Updated on 9/13/2006 Ø ® Y. CAI Florida International Chapter 3 ICPMS Advantages: ü Simplified chemical and physical separation procedures ü Elimination (reduction) of matrix effects ü Elimination of the effect of instrumental drift

Advanced Analytical Chemistry – CHM 6157 University Updated on 9/13/2006 Ø ® Y. CAI Florida International Chapter 3 ICPMS Theory In principle, any element with at least two isotopes that can be measured is suitable for determination by isotope dilution. The two selected are designed 1 and 2. Three solutions will be used: Sample (s) sample (m) Standard (t) Spiked

Advanced Analytical Chemistry – CHM 6157 University Updated on 9/13/2006 ü ü ü ü 1 n s ® Y. CAI Florida International Chapter 3 ICPMS is the number of moles of isotope 1 in the sample. 2 n is the number of moles of isotope 2 in the s sample. 1 n is the number of moles of isotope 1 in the t standard. 2 n is the number of moles of isotope 2 in the t standard. Rs is the ratio of isotope 1 to isotope 2 in the sample solution. Rt is the ratio of isotope 1 to isotope 2 in the standard. Rm is the ratio of isotope 1 to isotope 2 in the spiked sample.

Advanced Analytical Chemistry – CHM 6157 University Updated on 9/13/2006 ® Y. CAI Florida International Chapter 3 ICPMS Assuming the molecular sensitivity 1 S/2 S of the MS for isotope 1 and 2 are the same, then For the sample solution: Rs = 1 ns/2 ns [1] For the standard solution: Rt = 1 nt/2 nt [2]

Advanced Analytical Chemistry – CHM 6157 University Updated on 9/13/2006 ® Y. CAI Florida International Chapter 3 ICPMS For the spiked sample solution: Rm = (1 ns + 1 nt)/(2 ns + 2 nt) [3] Substitution of equations 1 and 2 into equation 3: Rm = (Rs 2 ns+ Rt 2 nt)/(2 ns + 2 nt) [4] Rearranged to: 2 n = 2 n (R -R )/(R -R ) [5] s t m t s m Convert the number of moles of isotope 2 in the sample to the total number of moles of the elements in the sample. ns =(2 nt/θ 2)(Rm-Rt)/(Rs-Rm) [6] θ 2 is the isotopic abundance of isotope 2 in the

Advanced Analytical Chemistry – CHM 6157 University Updated on 9/13/2006 ® Y. CAI Florida International Chapter 3 ICPMS The mass of the element in the sample is then given by: Ms = M(2 nt/θ 2)(Rm-Rt)/(Rs-Rm) [7] M is the molecular weight of the element.

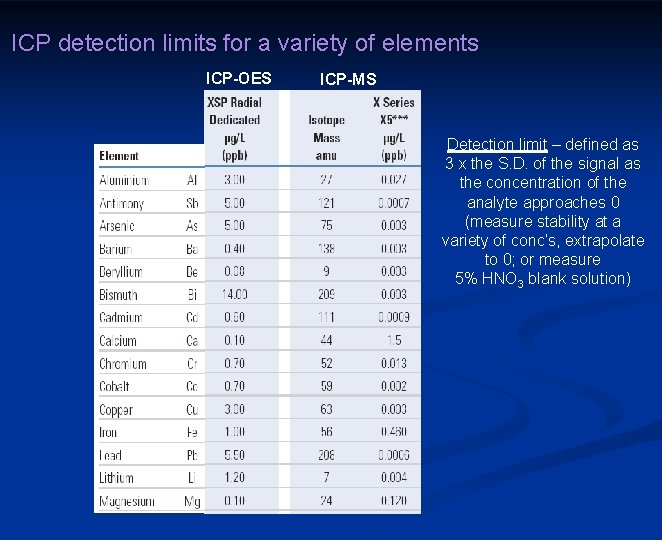
ICP detection limits for a variety of elements ICP-OES ICP-MS Detection limit – defined as 3 x the S. D. of the signal as the concentration of the analyte approaches 0 (measure stability at a variety of conc’s, extrapolate to 0; or measure 5% HNO 3 blank solution)

More Commonly used ICPMS terms Nebulization efficiency – the amount of solution that reaches the plasma (~1%) - varies with sample matrix - surface tension, viscosity, and density of solution will affect neb. eff. - usually all standards, spikes, and samples are introduced as 2 -5% HNO 3 - an acid solution reduces complexation, surface adsorption Matrix effects – the changes in ICP characteristics with variable matrices - largely black box (we see these effects, cannot wholly explain/predict them) - you must carefully match the matrices of your standards/samples to obtain quantitative results Ionization efficiency – the amount of ions produced per atoms introduced - depends on matrix, focusing of beam through cones, lenses - usually no better than 1/1000

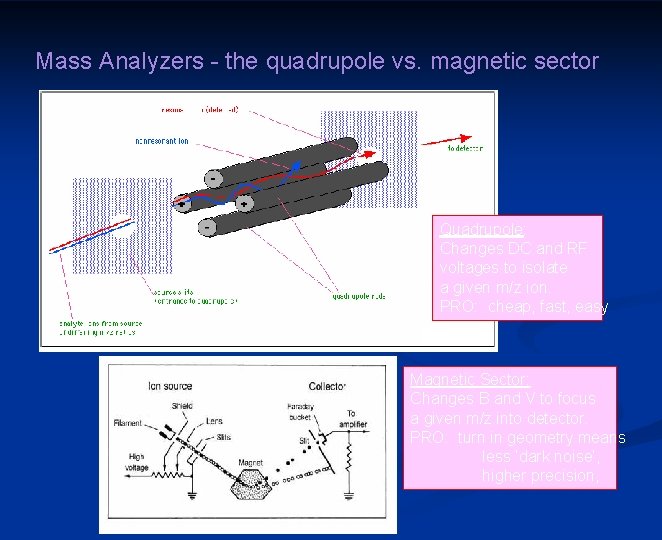
Mass Analyzers - the quadrupole vs. magnetic sector Quadrupole: Changes DC and RF voltages to isolate a given m/z ion. PRO: cheap, fast, easy Magnetic Sector: Changes B and V to focus a given m/z into detector. PRO: turn in geometry means less ‘dark noise’, higher precision,