Atomic Mass and Isotope Objectives SWBAT calculate the
Atomic Mass and Isotope Objectives: SWBAT calculate the average atomic mass of an element when given the relative abundance of the isotopes. Bellringer: What does the atomic mass tell you about the atom?

RELATIVE MASSES Chemists more than 200 years ago used a relative scale to compare weights of atoms to each other. Dalton assigned a H atom a mass of 1. According to his scale a helium atom has a relative mass of 4 because it is 4 times as heavy.

RELATIVE MASSES • Using Dalton’s scale a carbon atom has a relative mass of 12 because a carbon atom is twelve times heavier than a hydrogen atom

Comparing Masses • In 1961 Dalton’s method of comparing masses of atoms was replaced by IUPAC • International Union of Applied Physics and Chemistry.

IUPAC RELATIVE MASSES IUPAC decided that the most common isotope of C which is 12 C would be used as a reference standard and assigned an atom of 12 C a mass of 12 exactly. Using this scale the helium isotope is assigned a relative mass of 4 Comparing a helium atom to a carbon atom The He atom is 3 times lighter

RELATIVE ATOMIC MASSES A Krpton atom that is given a relative mass of 36 A Kr atom is three times heavier than a 12 C atom
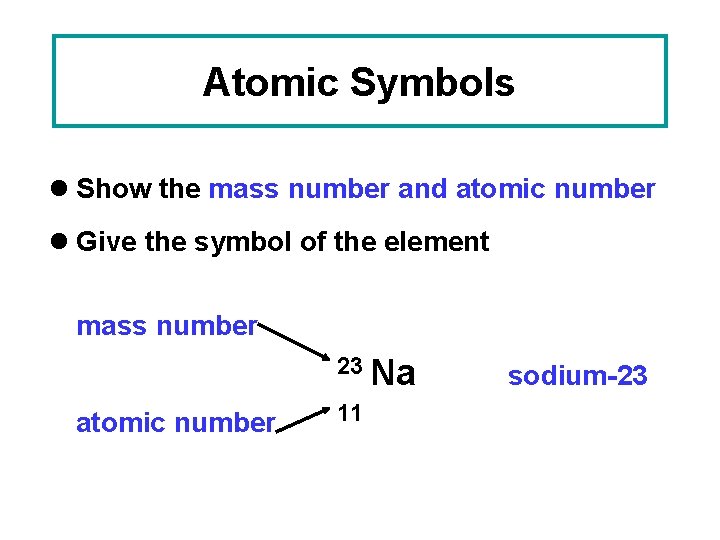
Atomic Symbols l Show the mass number and atomic number l Give the symbol of the element mass number 23 Na atomic number 11 sodium-23

Isotopes l Atoms with the same number of protons, but different numbers of neutrons. l Atoms of the same element (same atomic number) with different mass numbers Isotopes of chlorine 35 Cl 37 Cl 17 17 chlorine - 35 chlorine - 37

RELATIVE ATOMIC MASSES All isotopes of elements are given a relative isotopic mass compared to the 12 C isotope. There are 3 isotopes of Mg 24 Mg 25 Mg 26 Mg These 3 atoms are different because they have different numbers of neutrons

Abundances of Isotopes In a sample of pure Mg you will find the isotopes of Mg always occur in the following quantity 24 Mg 78. 7% 25 Mg 10. 13% 26 Mg 11. 17% Like Magnesium most elements exist as a mixture of isotopes. Eg 1 H, 2 H and 3 H
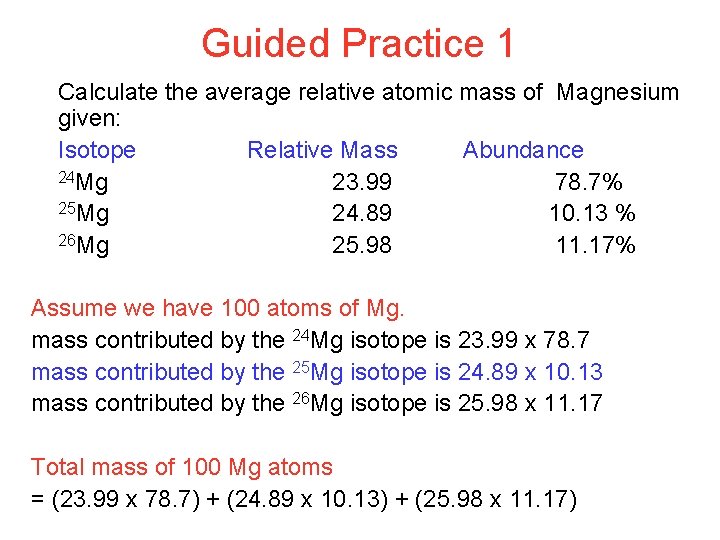
Guided Practice 1 Calculate the average relative atomic mass of Magnesium given: Isotope Relative Mass Abundance 24 Mg 23. 99 78. 7% 25 Mg 24. 89 10. 13 % 26 Mg 25. 98 11. 17% Assume we have 100 atoms of Mg. mass contributed by the 24 Mg isotope is 23. 99 x 78. 7 mass contributed by the 25 Mg isotope is 24. 89 x 10. 13 mass contributed by the 26 Mg isotope is 25. 98 x 11. 17 Total mass of 100 Mg atoms = (23. 99 x 78. 7) + (24. 89 x 10. 13) + (25. 98 x 11. 17)
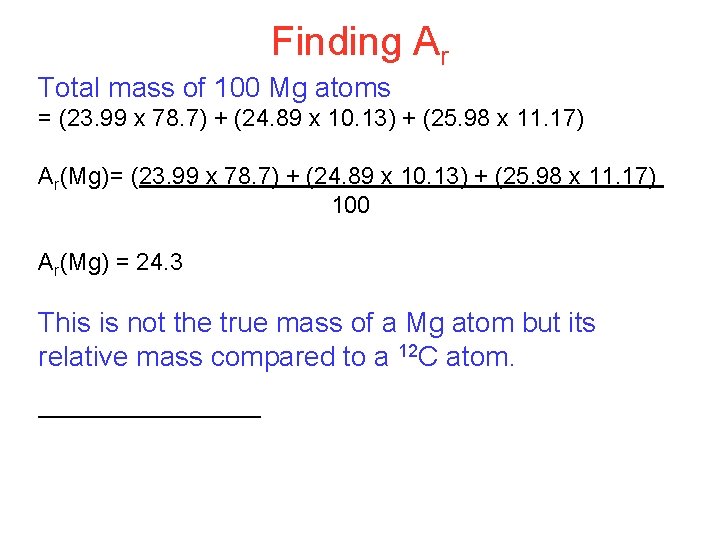
Finding Ar Total mass of 100 Mg atoms = (23. 99 x 78. 7) + (24. 89 x 10. 13) + (25. 98 x 11. 17) Ar(Mg)= (23. 99 x 78. 7) + (24. 89 x 10. 13) + (25. 98 x 11. 17) 100 Ar(Mg) = 24. 3 This is not the true mass of a Mg atom but its relative mass compared to a 12 C atom.

Finding Ar The general rule is: Ar = Σ(relative isotopic mass x abundance) 100

Guided Practice 2 • Find the relative atomic mass of Chorine. Isotope 35 Cl 37 Cl Relative Mass 34. 969 36. 966 Abundance 75. 80% 24. 20% Ar(Cl) = (34. 969 x 75. 8) + (36. 966 x 24. 2) 100 Ar(Cl) = 35. 45

Find Ar(O) Isotopes Relative Isotopic Mass 16 O 17 O 18 O 15. 995 16. 999 17. 999 Abundance 99. 76% 0. 04% 0. 20% Ar(O) = (15. 995 x 99. 76) + (16. 999 x 0. 04) + (17. 999 x 0. 2) 100 Ar(O) = 16
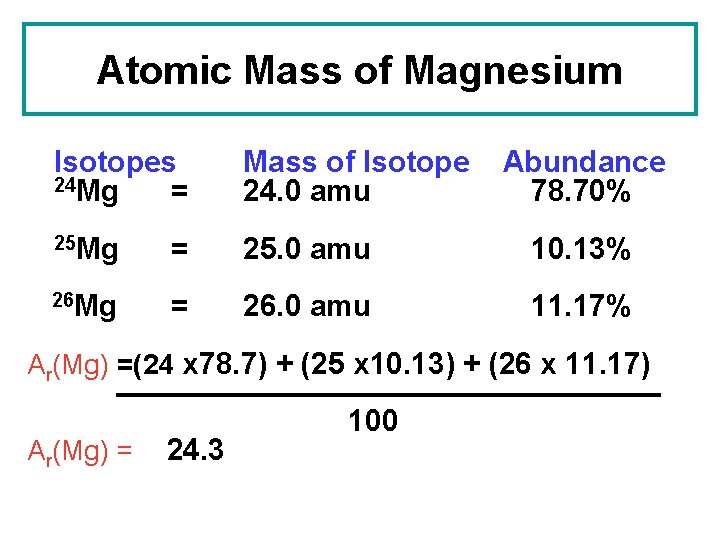
Atomic Mass of Magnesium Isotopes 24 Mg = Mass of Isotope 24. 0 amu Abundance 78. 70% 25 Mg = 25. 0 amu 10. 13% 26 Mg = 26. 0 amu 11. 17% Ar(Mg) =(24 x 78. 7) + (25 x 10. 13) + (26 x 11. 17) Ar(Mg) = 24. 3 100

HW: Practice • Quiz tomorrow on: – Atom History – Atomic Structure – Drawing Bohr Models – Finding Relative Abundance (? )
- Slides: 17