Atomic History The Nuts and Bolts of Chemistry

Atomic History The Nuts and Bolts of Chemistry – The Missing Manual of Historical Figures

OVERVIEW • • • Early Greeks - Democritus v Aristotle Dalton’s Atomic Theory Thomson - discovery of electrons Rutherford - discovery of nucleus Chadwick - discovery of neutron Bohr - planetary model of atom ket 09232004

OVERVIEW • • • Early Greeks - Democritus v Aristotle Dalton’s Atomic Theory Thomson - discovery of electrons Rutherford - discovery of nucleus Chadwick - discovery of neutron Bohr - planetary model of atom ket 09232004

Early Greeks • • What is the nature of matter? -Infinitely divisible pieces of “stuff” -Earth, Air, Fire , and Water No experiments ket 09232004

Early Greeks ket 09232004
Aristotle vs. Democritus • Democritus • Aristotle • New idea • Didn’t buy it… • “atomos” - indivisible • All things are infinitely divisible • Eventually, can’t divide matter any more • Guess who won? • First “atomic theory” ket 09232004

(Aristotle) For 2000 years, scientists thought all matter was infinitely divisible ket 09232004

OVERVIEW • • • Early Greeks - Democritus v Aristotle Dalton’s Atomic Theory Thomson - discovery of electrons Rutherford - discovery of nucleus Chadwick - discovery of neutron Bohr - planetary model of atom ket 09232004

Dalton’s Atomic Theory John Dalton • 1807 • Idea of “atom” – Solid spheresindestructible – Unique to each element – Combine evenly – Reactions are rearrangements ket 09232004
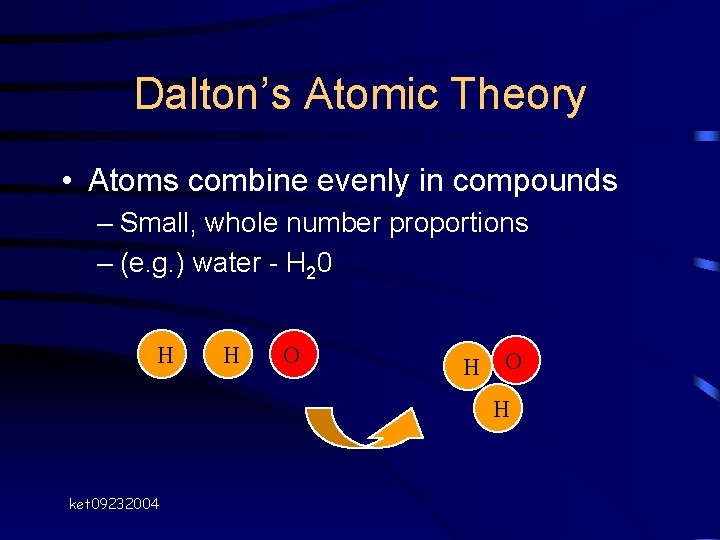
Dalton’s Atomic Theory • Atoms combine evenly in compounds – Small, whole number proportions – (e. g. ) water - H 20 H H O H ket 09232004

OVERVIEW • • • Dalton’s Atomic Theory Thomson - discovery of electrons Rutherford - discovery of nucleus Chadwick - discovery of neutron Bohr - planetary model of atom ket 09232004

Thomson’s Discovery John Dalton • Cathode Ray Tube – Sends a “ray” of particles • Used magnet to deflect beam first • So, beam was made of particles • Thomson then passed ray through +/- charged plates J. J. Thomson 1897 ket 09232004
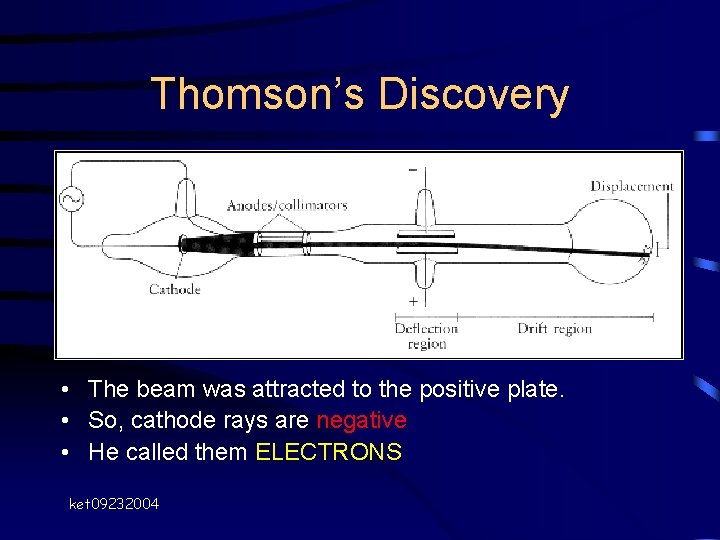
Thomson’s Discovery • The beam was attracted to the positive plate. • So, cathode rays are negative • He called them ELECTRONS ket 09232004

Thomson’s Discovery • Most books give Thomson credit for discovering proton • He and Millikan found the mass of an electron to be much smaller than an atom • So, electrons are VERY small • Protons must be large in comparison – plum pudding model ket 09232004
Plum Pudding model Preface: “Plum Pudding” atomic model • atoms are solid • made of positively-charged material • with negative “bits” scattered throughout (like raisins in plum pudding) (or raisin bread) ket 09232004

OVERVIEW • • • Dalton’s Atomic Theory Thomson - discovery of electrons Rutherford - discovery of nucleus Chadwick - discovery of neutron Bohr - planetary model of atom ket 09232004

Rutherford’s Discovery Ernest Rutherford ket 09232004 Image courtesty of http: //www. nobel. se/chemistry/laureates/1908/rutherford-bio. html
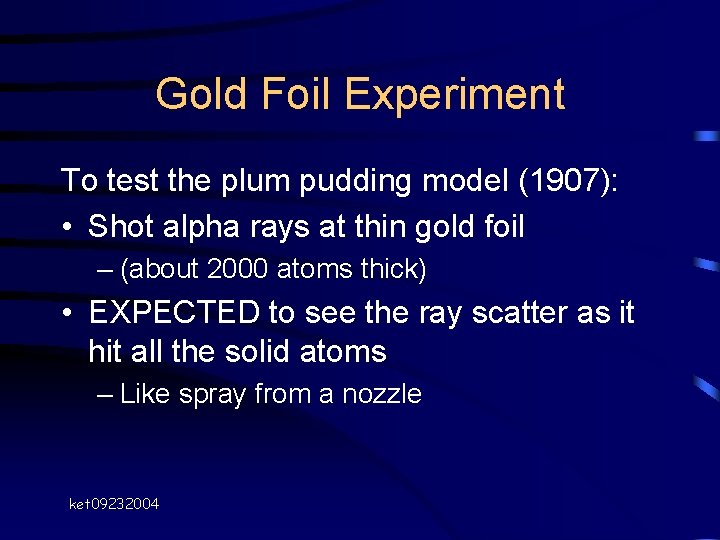
Gold Foil Experiment To test the plum pudding model (1907): • Shot alpha rays at thin gold foil – (about 2000 atoms thick) • EXPECTED to see the ray scatter as it hit all the solid atoms – Like spray from a nozzle ket 09232004
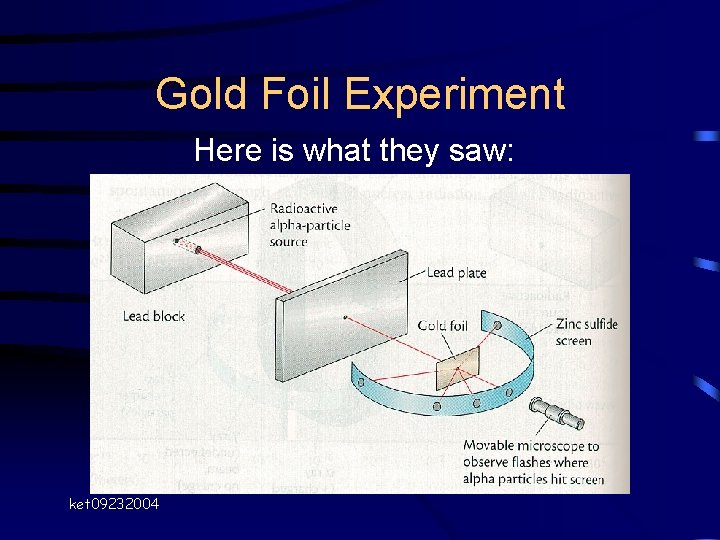
Gold Foil Experiment Here is what they saw: ket 09232004
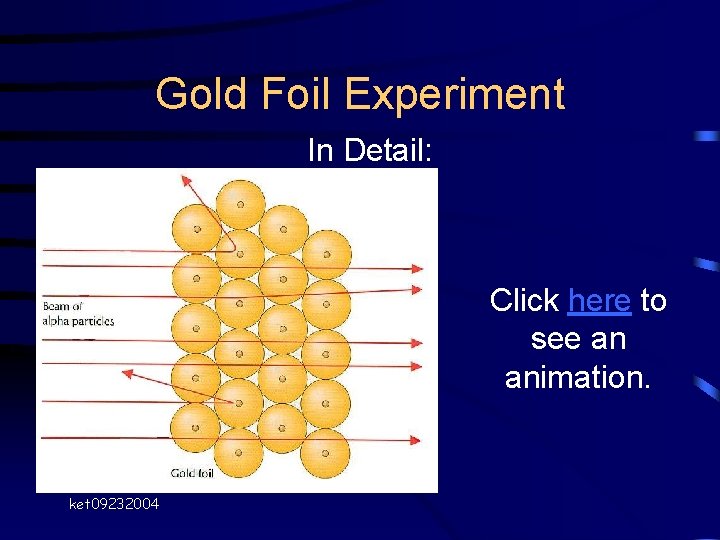
Gold Foil Experiment In Detail: Click here to see an animation. ket 09232004

Gold Foil Experiment RESULTS • Most of the particles were not deflected • Some were minimally deflected • VERY few (1 in 20, 000) bounced back – “as if you had fired a 15 -inch shell at a piece of tissue paper and it came back and hit you. ” - Rutherford ket 09232004

Gold Foil Experiment CONCLUSIONS • Plum pudding model wrong • A “nucleus” exists – It is tiny – It is densely-packed and positively-charged • Empty spaces exist in atoms – LOTS of it!!!! ket 09232004

Gold Foil Experiment How much empty space? • Use a billiard ball to represent a nucleus • The electrons occupy a volume one kilometer in ALL DIRECTIONS • Most of that space is EMPTY. ket 09232004

OVERVIEW • • • Dalton’s Atomic Theory Thomson - discovery of electrons Rutherford - discovery of nucleus Chadwick - discovery of neutron Bohr - planetary model of atom ket 09232004

Chadwick’s Discovery (1932) Chadwick ket 09232004 • PROBLEM • There was more mass in nucleus than explained by protons alone • Where did it come from? • NEUTRONS

OVERVIEW • • • Dalton’s Atomic Theory Thomson - discovery of electrons Rutherford - discovery of nucleus Chadwick - discovery of neutron Bohr - planetary model of atom ket 09232004
Bohr’s Atomic Model • Nucleus has + charge • Electrons have charge • Why don’t electrons simply “fall into” the nucleus? Bohr ket 09232004
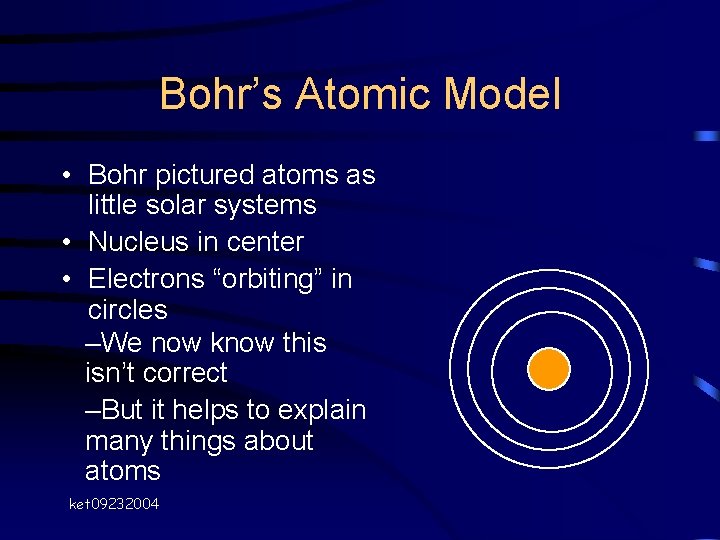
Bohr’s Atomic Model • Bohr pictured atoms as little solar systems • Nucleus in center • Electrons “orbiting” in circles –We now know this isn’t correct –But it helps to explain many things about atoms ket 09232004

HOMEWORK • Read: • Do: ket 09232004 Section 3. 2 p. 89; Q’s 1 -3 p. 107; Q’s 17, 18
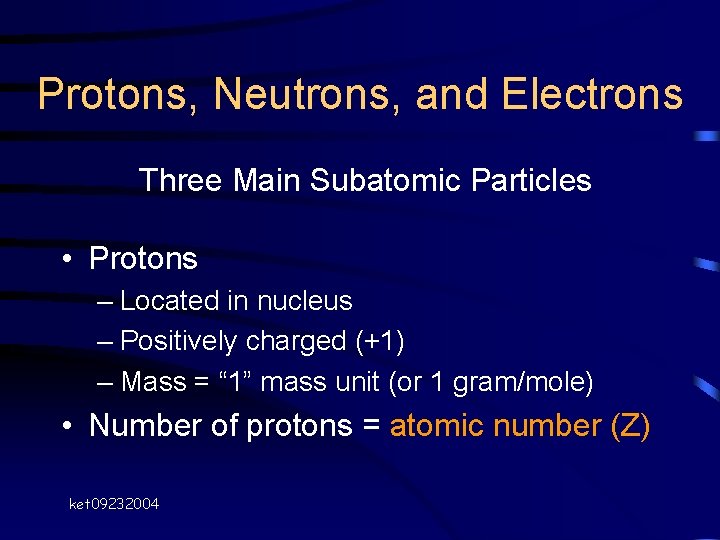
Protons, Neutrons, and Electrons Three Main Subatomic Particles • Protons – Located in nucleus – Positively charged (+1) – Mass = “ 1” mass unit (or 1 gram/mole) • Number of protons = atomic number (Z) ket 09232004
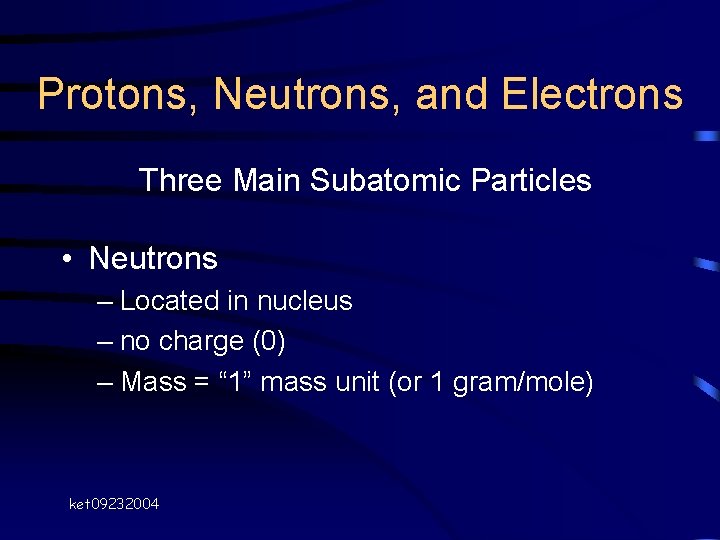
Protons, Neutrons, and Electrons Three Main Subatomic Particles • Neutrons – Located in nucleus – no charge (0) – Mass = “ 1” mass unit (or 1 gram/mole) ket 09232004
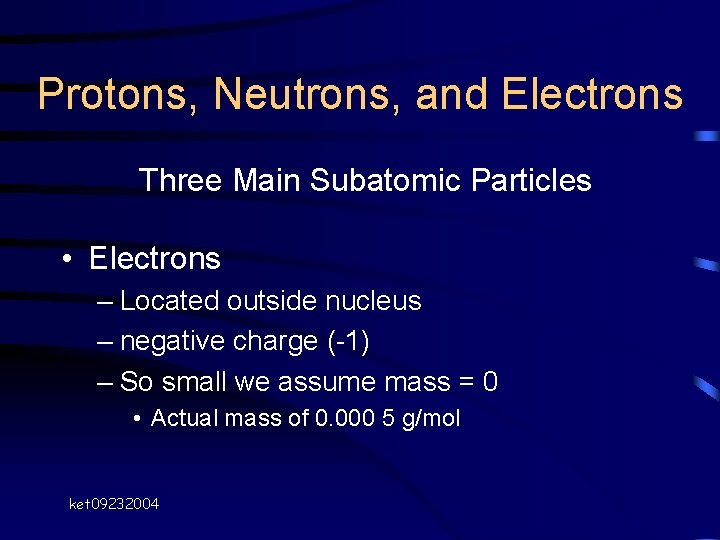
Protons, Neutrons, and Electrons Three Main Subatomic Particles • Electrons – Located outside nucleus – negative charge (-1) – So small we assume mass = 0 • Actual mass of 0. 000 5 g/mol ket 09232004

Protons, Neutrons, and Electrons Atomic number (Z) • shown in lower left • ALWAYS equals the number of protons • Equals the number of electrons in a neutral atom ket 09232004 Isotope Notation C 6
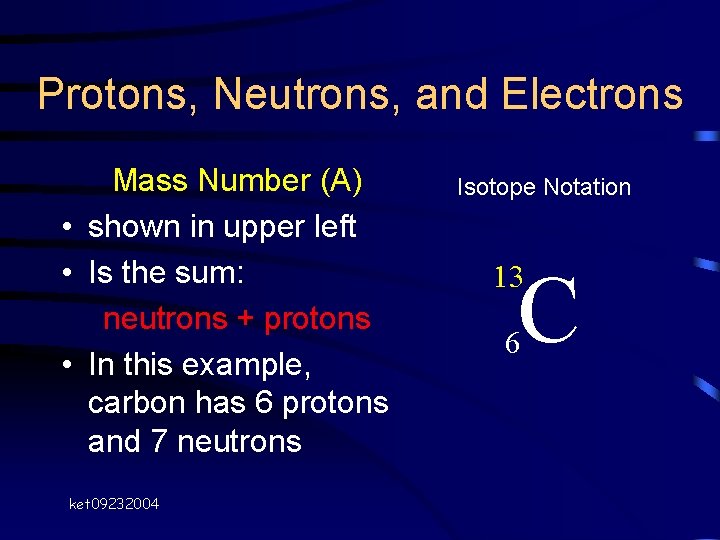
Protons, Neutrons, and Electrons Mass Number (A) • shown in upper left • Is the sum: neutrons + protons • In this example, carbon has 6 protons and 7 neutrons ket 09232004 Isotope Notation C 13 6

Protons, Neutrons, and Electrons We call this atom “carbon-13” Isotope Notation C 13 6 ket 09232004

Protons, Neutrons, and Electrons • Atoms with the same number of protons can have different numbers of neutrons. • We call such atoms isotopes ket 09232004
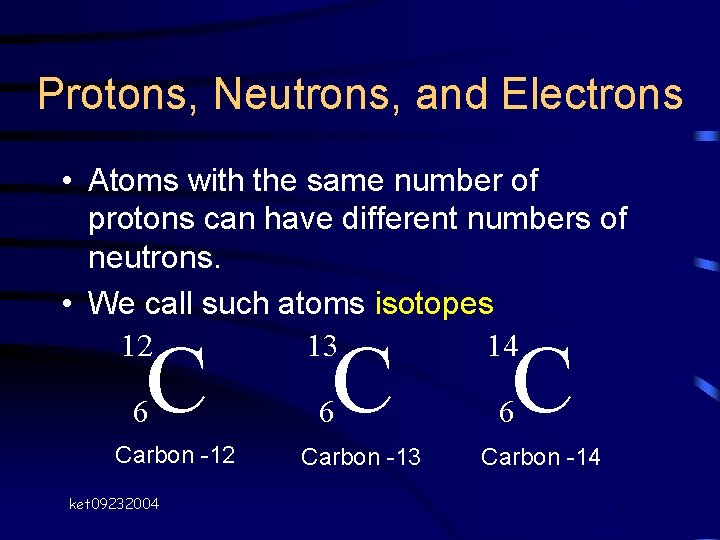
Protons, Neutrons, and Electrons • Atoms with the same number of protons can have different numbers of neutrons. • We call such atoms isotopes 12 13 14 C 6 Carbon -12 ket 09232004 C 6 Carbon -13 C 6 Carbon -14
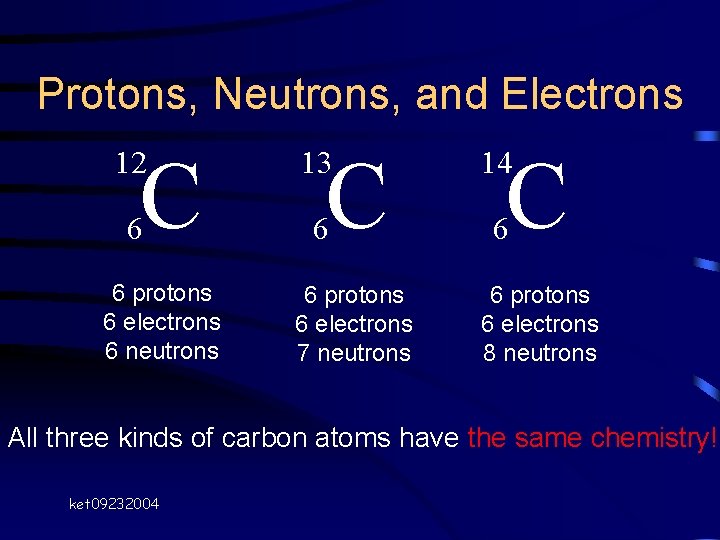
Protons, Neutrons, and Electrons C C C 12 13 14 6 6 protons 6 electrons 6 neutrons 6 protons 6 electrons 7 neutrons 6 protons 6 electrons 8 neutrons All three kinds of carbon atoms have the same chemistry! ket 09232004
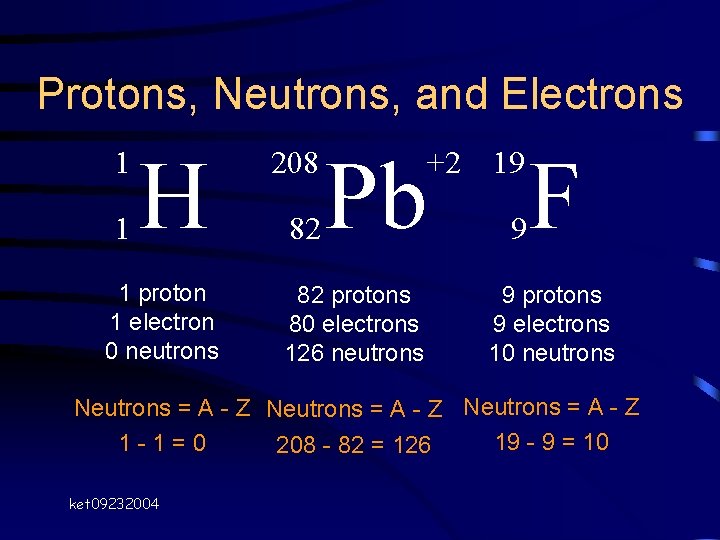
Protons, Neutrons, and Electrons 1 1 H 1 proton 1 electron 0 neutrons 208 82 Pb 82 protons 80 electrons 126 neutrons +2 19 9 F 9 protons 9 electrons 10 neutrons Neutrons = A - Z 19 - 9 = 10 1 -1=0 208 - 82 = 126 ket 09232004

Protons, Neutrons, and Electrons 7 3 ? What element is this? How do you know? ket 09232004

Protons, Neutrons, and Electrons 7 3 Li What element is this? Lithium How do you know? the atomic number is 3 look on the periodic table ket 09232004
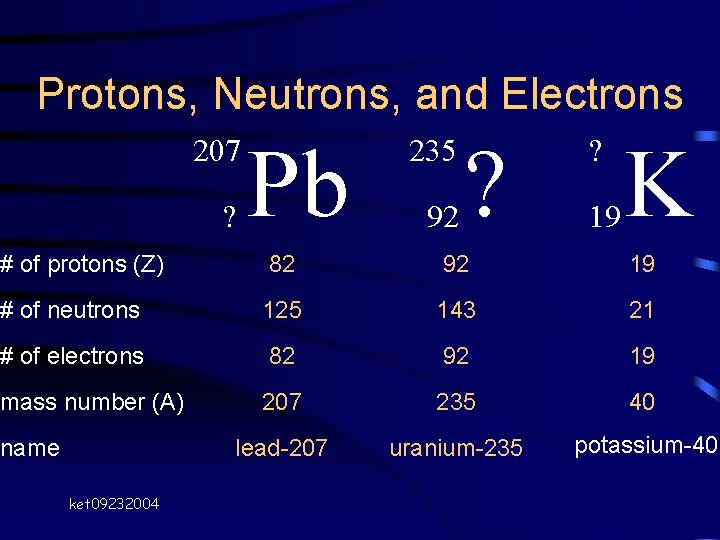
Protons, Neutrons, and Electrons 207 ? Pb 235 92 ? ? 19 K # of protons (Z) 82 92 19 # of neutrons 125 143 21 # of electrons 82 92 19 mass number (A) 207 235 40 lead-207 uranium-235 potassium-40 name ket 09232004
- Slides: 42