Atomic Emission Spectroscopy Lecture 18 Arcs Sparks And




























- Slides: 87

Atomic Emission Spectroscopy Lecture 18 Arcs, Sparks And Plasma 1

Atomic Emission Spectroscopy (AES) - (AES), in contrast to AAS, uses the very high temperatures of atomization sources to excite atoms. - Thus excluding the need for lamp sources. Emission sources, which are routinely used in AES: 1) plasma, 2) arcs and 3) sparks, 4) flames. We will study the different types of emission sources, their operational principles, features, and operational characteristics. Finally, instrumental designs and applications of emission methods will be discussed. 2

Plasma Sources The term “plasma” is defined as a homogeneous mixture of (gaseous atoms, ions and electrons) at very high temperatures. Two types of plasma atomic emission sources are frequently used: 1. Inductively coupled plasma (ICP). 2. Direct current plasma (DCP). 3

4

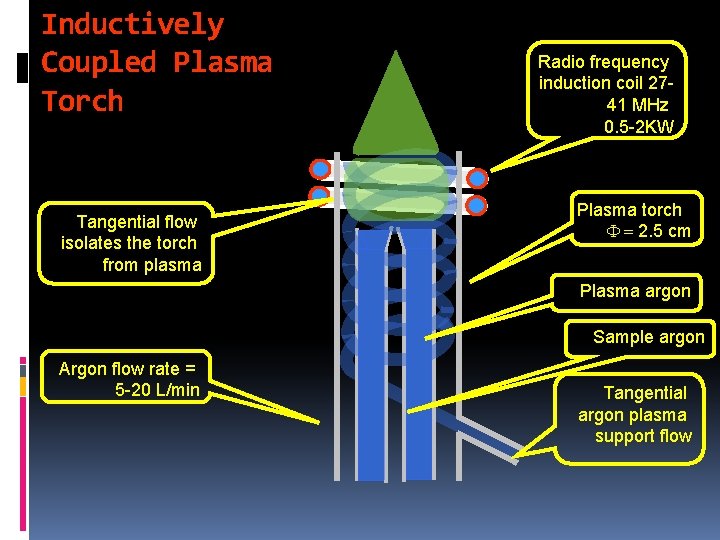
Inductively Coupled Plasma (ICP) A typical ICP consists: - Three concentric quartz tubes ( )ﻣﺘﺪﺍﺧﻼﺕ ﺑﻨﻔﺲ ﺍﻟﻤﺤﻮﺭ through which streams of argon gas flow at a rate in the range from 5 -20 L/min. - The outer tube is about 2. 5 cm (1 inch) in diameter and the top of this tube is surrounded by a radiofrequency powered induction coil (RF) producing a power of about 2 k. W at a frequency in the range from 27 -41 MHz. - This coil produces a strong magnetic field as well. 5

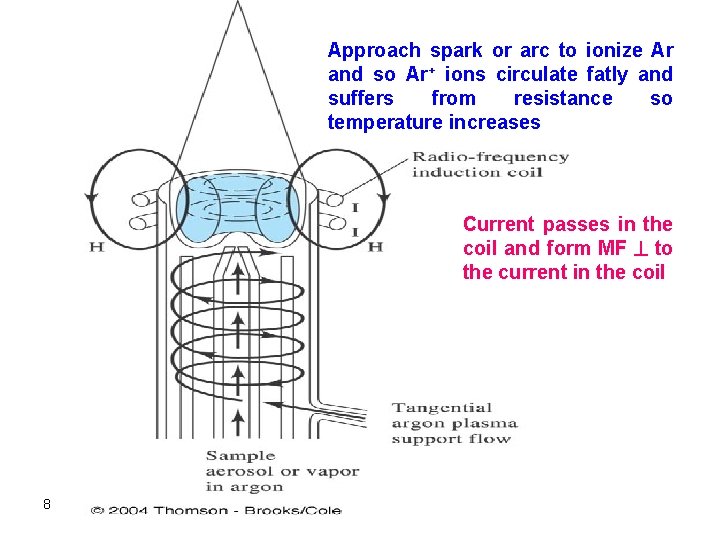
- Ionization of flowing argon is achieved by a spark. - The ionized argon interacts with the strong magnetic field and is thus forced to move within the vicinity of the induction coil at a very high speed. - A very high temperature is obtained as a result of the very high resistance experienced by circulating argon (collisions of e- and cations with ambient gas) (ohmic heating). - Why Ar used? ? : 1 - inert 2 - few emission lines 6

- The top of the quartz tube will experience very high temperatures and should, therefore, be isolated and cooled. Cooling: This can be accomplished by passing argon tangentially around the walls of the tube. A schematic of an ICP (usually called a torch plasma) is shown below: 7

Approach spark or arc to ionize Ar and so Ar+ ions circulate fatly and suffers from resistance so temperature increases Current passes in the coil and form MF to the current in the coil 8

Inductively Coupled Plasma Torch Tangential flow isolates the torch from plasma Radio frequency induction coil 2741 MHz 0. 5 -2 KW Plasma torch F = 2. 5 cm Plasma argon Sample argon Argon flow rate = 5 -20 L/min Tangential argon plasma support flow

The torch is formed as a result of the argon emission at the very high temperature of the plasma. The temperature gradients in the ICP torch can be pictured in the following graphics: 10

11

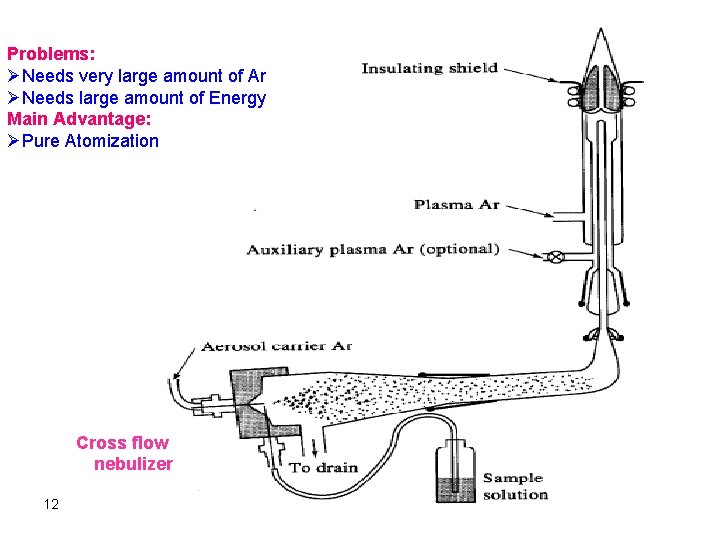
Problems: ØNeeds very large amount of Ar ØNeeds large amount of Energy Main Advantage: ØPure Atomization Cross flow nebulizer 12

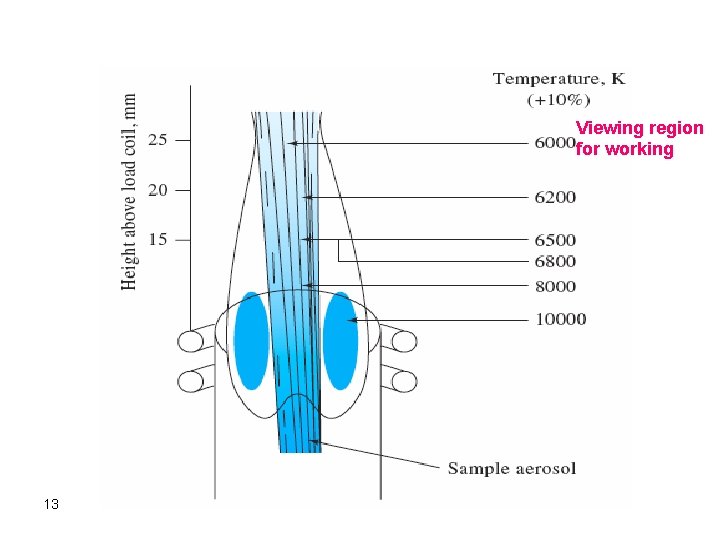
Viewing region for working 13

v The viewing region: Used in elemental analysis is usually about 6000 o. C, which is about 1. 5 -2. 5 cm above the top of the tube. v High cost of the ICP torch: Because argon consumption is relatively high which makes the running v Argon is a unique inert gas for plasma torches: Since it has few emission lines. This decreases possibility of interferences with other analyte lines. 14

Sample Introduction Several methods for sample introduction: The most widely used is: The nebulization of an analyte solution into the plasma. However, other methods, as described earlier, are fine where vapors of analyte molecules or atom from electrothermal or ablation devices can be driven into the torch for complete atomization and excitation. For your convenience, sample introduction methods are summarized here again: 15

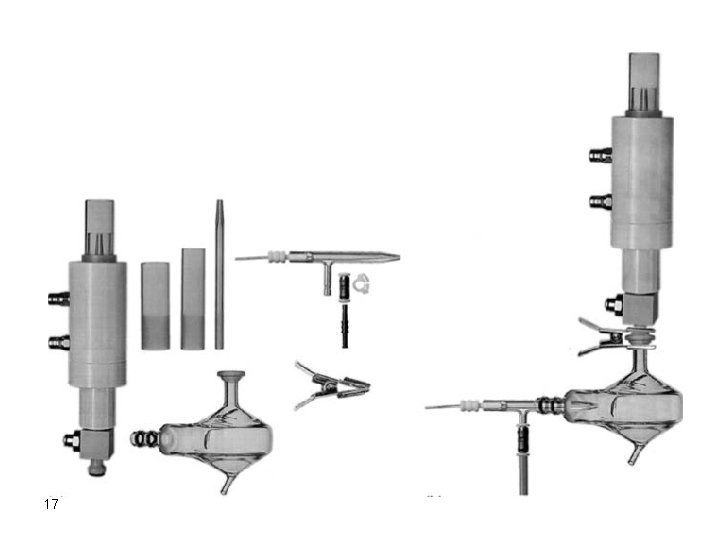
Samples in Solution Pneumatic Nebulizers Ø Samples in solution are usually easily introduced into the atomizer by a simple nebulization, aspiration, process. Ø Nebulization converts the solution into an aerosol of very fine droplets using a jet of compressed gas. Ø The flow of gas carries the aerosol droplets to the atomization chamber or region. 16

17

Ultrasonic Nebulizers ØIn this case samples are pumped onto the surface of a piezoelectric crystal that vibrates in the k. Hz to MHz range. Ø Such vibrations convert samples into homogeneous aerosols that can be driven into atomizers. ØUltrasonic nebulization is preferred over pneumatic nebulization since finer droplets and more homogeneous aerosols are usually achieved. Ø However, most instruments use pneumatic nebulization for convenience. 18

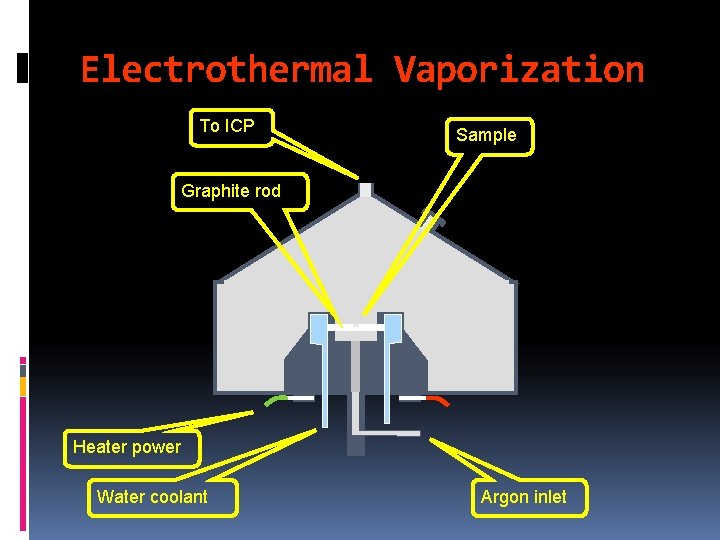
Electrothermal Vaporization (Only for sample introd. Not for atomization) ØAn accurately measured quantity of sample (few m. L) is introduced into an electrically heated cylindrical chamber through which an inert gas flows. Ø Usually, the cylinder is made of pyrolytic carbon but tungsten cylinders are now available. ØThe vapors of molecules and atoms are swept into the plasma source for complete atomization and excitation. 19

Electrothermal Vaporization To ICP Sample Graphite rod Heater power Water coolant Argon inlet

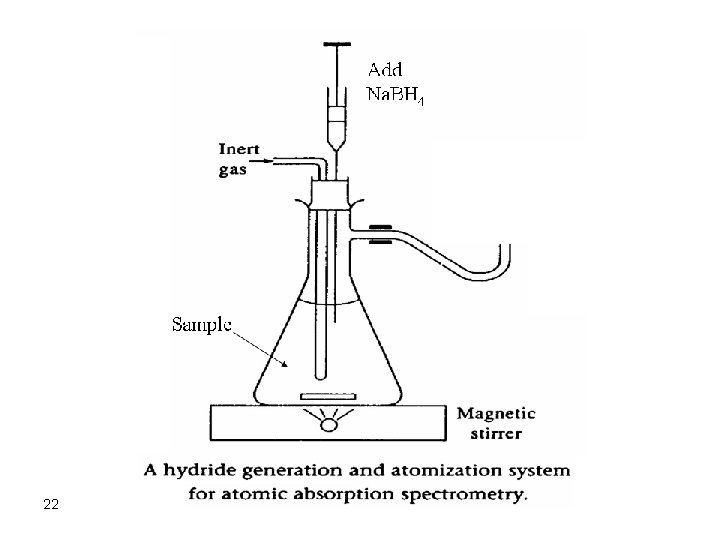
Hydride Generation Techniques Ø Samples that contain arsenic, antimony, tin, selenium, bismuth, and lead can be vaporized by converting them to volatile hydrides by addition of sodium borohydride. Ø Volatile hydrides are then swept into the plasma by a stream of an inert gas. 21

22

Introduction of Solid Samples A variety of techniques were used to introduce solid samples into atomizers. These include: 1. Conductive Samples Ø If the sample is conductive and is of a shape that can be directly used as an electrode (like a piece of metal or coin), that would be the choice for sample introduction in arc and spark techniques. Ø Otherwise, powdered solid samples are mixed with fine graphite and made into a paste. Ø Upon drying, this solid composite can be used as an electrode. Ø The discharge caused by arcs and sparks interacts with the surface of the solid sample creating a plume of very fine particulates and atoms that are swept into the plasma by argon flow. 23

Laser Ablation Ø Sufficient energy from a focused intense laser will interact with the surface of samples (in a similar manner like arcs and sparks) resulting in ablation. Ø The vapors of molecules and atoms are swept into the plasma source for complete atomization and excitation. Ø Laser ablation is becoming increasingly used since it is applicable to conductive and nonconductive samples. 24

The Glow Discharge Technique The technique is used for sample introduction and atomization as well. Ø The electrodes are kept at a 250 to 1000 V DC. Ø This high potential is sufficient to cause ionization of argon, which will be accelerated to the cathode where the sample is introduced. Ø Collision of the fast moving energetic argon ions with the sample (cathode) causes atomization by a process called sputtering. Ø Samples should thus be conductive to use the technique of glow discharge. Ø The vapors of molecules and atoms are swept into the plasma source for complete atomization and excitation by flowing argon. Ø However, nonconductive samples were reported to be atomized by this technique where they were mixed with a conductor material like graphite or 25 powdered copper.

Plasma Appearance and Spectra Ø A plasma torch looks very much like a flame but with a very intense nontransparent brilliant ﻻﻣﻊ white color at the core (less than 1 cm above the top of the tube). Ø In the region from 1 -3 cm above the top of the tube, the plasma becomes transparent. Ø The temperatures used are at least two to three orders of magnitude higher than that achieved by flames which may suggest efficient atomization and fewer chemical interferences. 26

Ionization in plasma is not a problem: It is may be thought to be a problem due to the very high temperatures But fortunately: the large electron flux from the ionization of argon will suppress ionization of all species. 27

2) The Direct Current Plasma (DCP) Ø The DCP is composed of three electrodes arranged in an inverted Y configuration. - A tungsten cathode resides at the top arm of the inverted Y. - The lower two arms are occupied by two graphite anodes. Ø Argon flows from the two anode blocks and plasma is obtained by momentarily bringing the cathode in contact with the anodes. Ø Argon ionizes and a high current passes through the cathode and anodes. 28

ØIt is this current which ionizes more argon and sustains the current indefinitely. Ø Samples are aspirated into the vicinity of the electrodes (at the center of the inverted Y) where the temperature is about 5000 o. C. ØDCP sources usually have fewer lines than ICP sources, require less argon/hour, and have lower sensitivities than ICP sources. Ø In addition, the graphite electrodes tend to decay with continuous use and should thus be frequently exchanged. A schematic of a DCP source is shown below: 29

30

31

DCP advantages: - Less argon consumption about 1/3 the ICP and less E of power supply. - Simpler instrumental requirements. - less spectral line interference (lower atomization temp. about 5000 0 C). However: ICP sources are more convenient to work with: - ICP is free from frequent consumables (like the anodes in DCP’s which need to be frequently changed) - More sensitive than DCP sources. 32

Advantages of Plasma Sources 1. No oxide formation as a result of two factors including: • Very high temperature • Inert environment inside the plasma (no oxygen) 2. Minimum chemical interferences (no or few ionization) e’s from the ionized Ar suppress ioniztion. 3. Minimum spectral interferences except for higher possibility of spectral line interference due to exceedingly large number of emission lines (because of high temperature) 33

4. Uniform temperature which results in precise determinations 5. No self-absorption is observed which extends the linear dynamic range to higher concentrations 6. No need for a separate lamp for each element 7. Easily adaptable to multichannel analysis (simultaneous measurements of many elements). 34

Plasma Emission Instruments Three classes of plasma emission instruments can be presented including: 1. Sequential instruments ﻗﻴﺎﺱ ﺗﺘﺎﺑﻌﻲ In this class of instruments a single channel detector is used: Ø The signal for each element is read using the specific wavelength for each element sequentially. Ø each element is measured after the another. Two types of sequential instruments are available: 35

a. Linear sequential scan instruments: ﺑﻨﻔﺲ ﺍﻟﺴﺮﻋﺔ The wavelength is linearly changed with time. Therefore, the grating is driven by a single speed during an analysis of interest. b. Slew scan instruments: The monochromator is preset to provide specific wavelengths: - moving very fast in between wavelengths. - while moving slowly at the specific wavelengths. Therefore, a two-speed motor driving the grating is thus used. 36

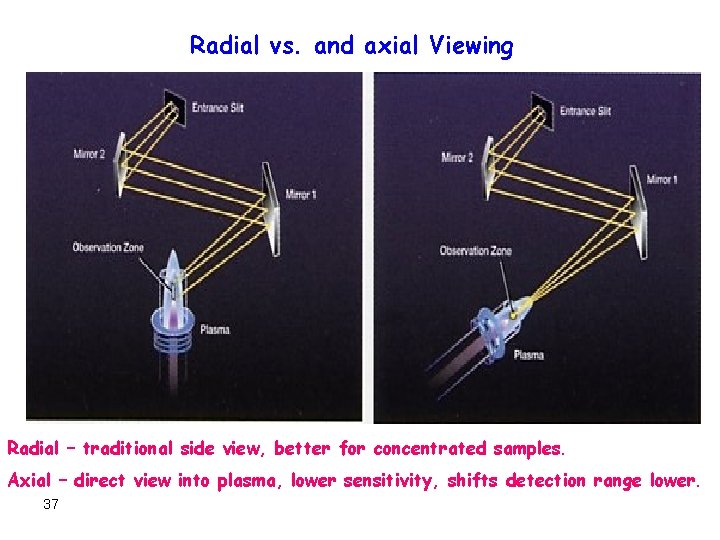
Radial vs. and axial Viewing Radial – traditional side view, better for concentrated samples. Axial – direct view into plasma, lower sensitivity, shifts detection range lower. 37

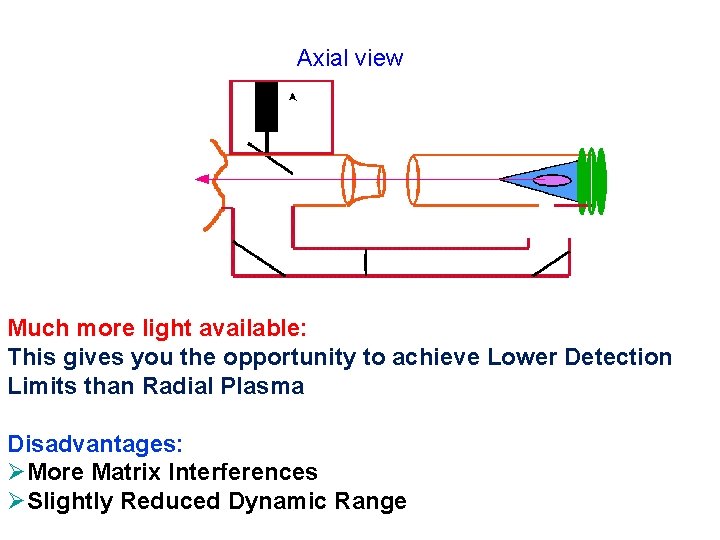
Axial view Much more light available: This gives you the opportunity to achieve Lower Detection Limits than Radial Plasma Disadvantages: ØMore Matrix Interferences ØSlightly Reduced Dynamic Range

Radial view 39

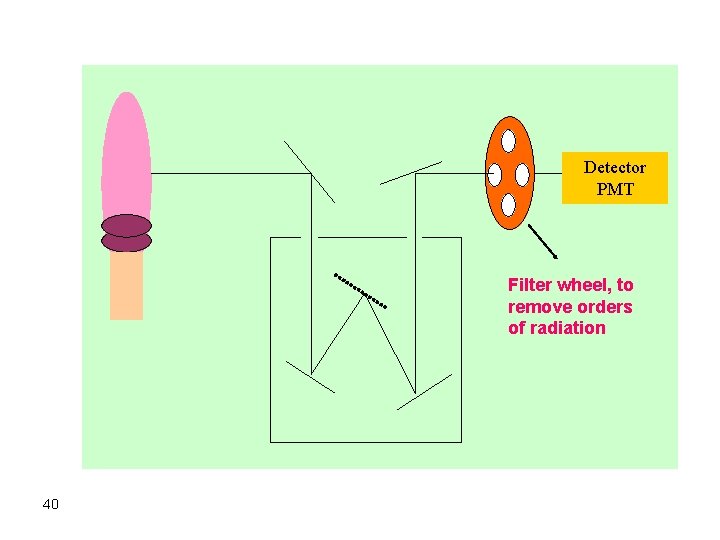
Detector PMT Filter wheel, to remove orders of radiation 40

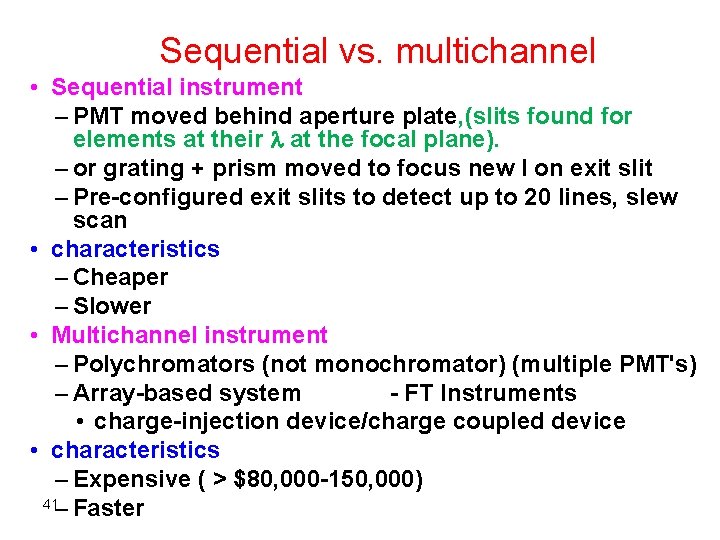
Sequential vs. multichannel • Sequential instrument – PMT moved behind aperture plate, (slits found for elements at their at the focal plane). – or grating + prism moved to focus new l on exit slit – Pre-configured exit slits to detect up to 20 lines, slew scan • characteristics – Cheaper – Slower • Multichannel instrument – Polychromators (not monochromator) (multiple PMT's) – Array-based system - FT Instruments • charge-injection device/charge coupled device • characteristics – Expensive ( > $80, 000 -150, 000) 41– Faster

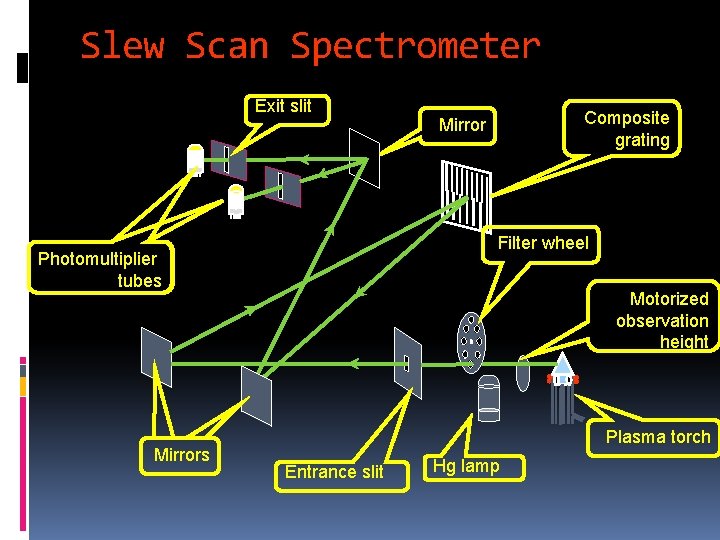
Slew scan spectrometer • Two slew-scan gratings • Two PMTs for VIS and UV • Most use holographic grating 42

Slew Scan Spectrometer Exit slit Filter wheel Photomultiplier tubes Mirrors Composite grating Mirror Motorized observation height Plasma torch Entrance slit Hg lamp

2. Multichannel Instruments This class of instruments is also referred to as simultaneous instruments in which all signals are reported at the same time using two types of configurations: 44

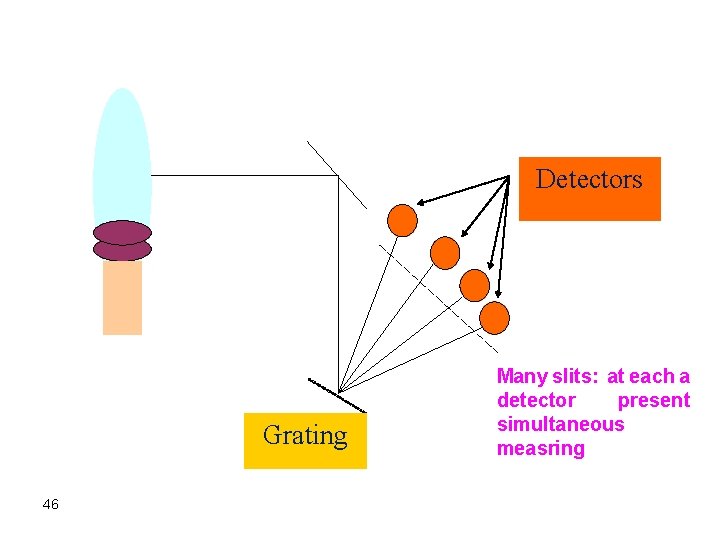
a. Polychromators (do not confused with monochromator) Multiple detectors: each measure 1 Ø Usually photomultiplier tubes are used. Ø Beams of grating are representing element) are detection. radiation emerging from the guided to exit slits (each the wavelength of a specific focused at several PMTs for Ø Detection, thus, takes place simultaneously 45

Detectors Grating 46 Many slits: at each a detector present simultaneous measring

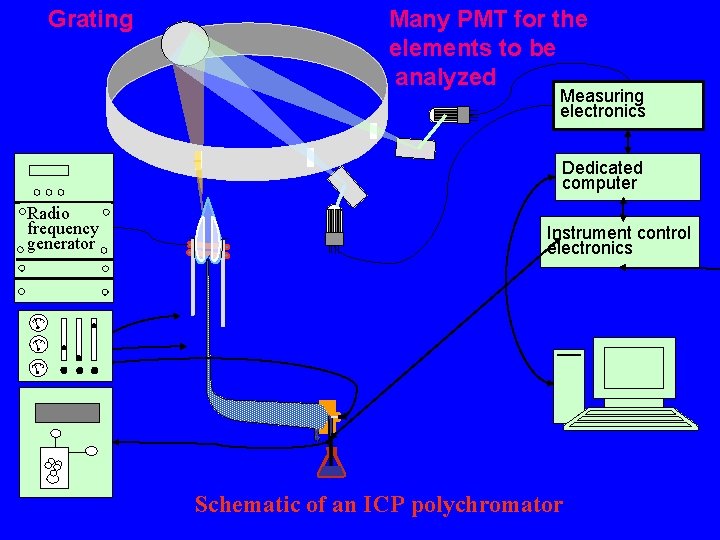
Grating Many PMT for the elements to be analyzed Measuring electronics Dedicated computer Radio frequency generator Instrument control electronics Schematic of an ICP polychromator

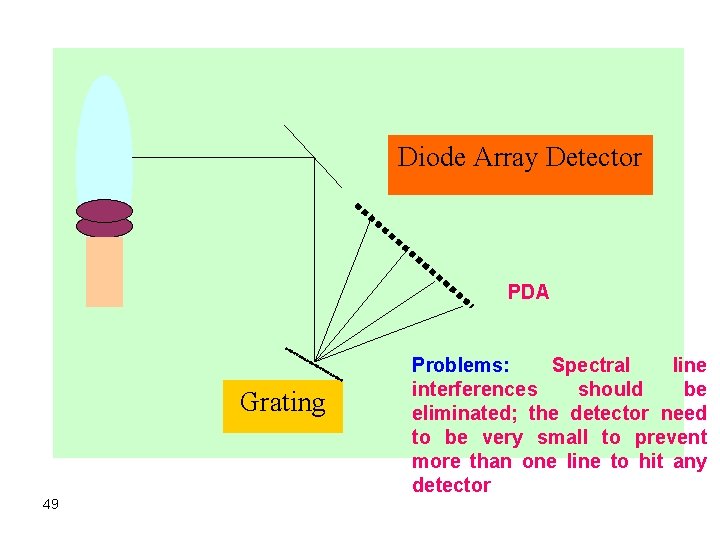
b. Array-based systems Ø This multichannel type instrument uses a multichannel detector like a charge injection device or a charge-coupled device. Ø Diffracted beams from a grating pass through a prism where further resolution of diffracted beams takes place by a prism. Ø The prism will disperse the orders of each diffracted beam. Ø The multichannel detector can also be a linear photodiode array as in the figure 48 below:

Diode Array Detector PDA Grating 49 Problems: Spectral line interferences should be eliminated; the detector need to be very small to prevent more than one line to hit any detector

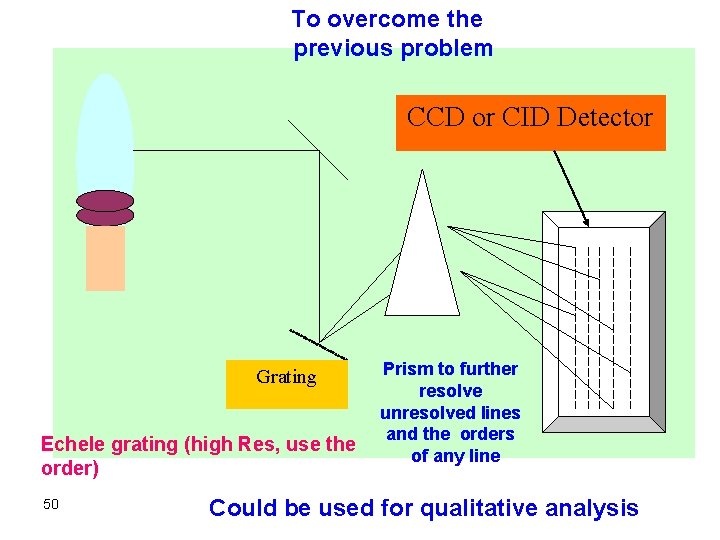
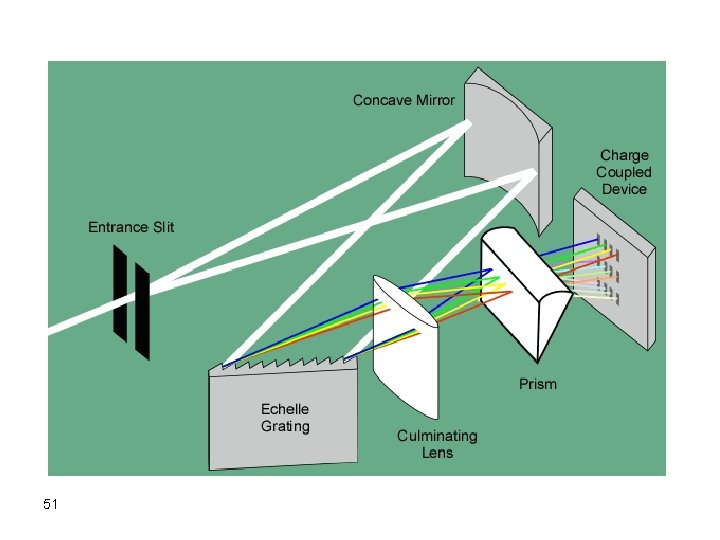
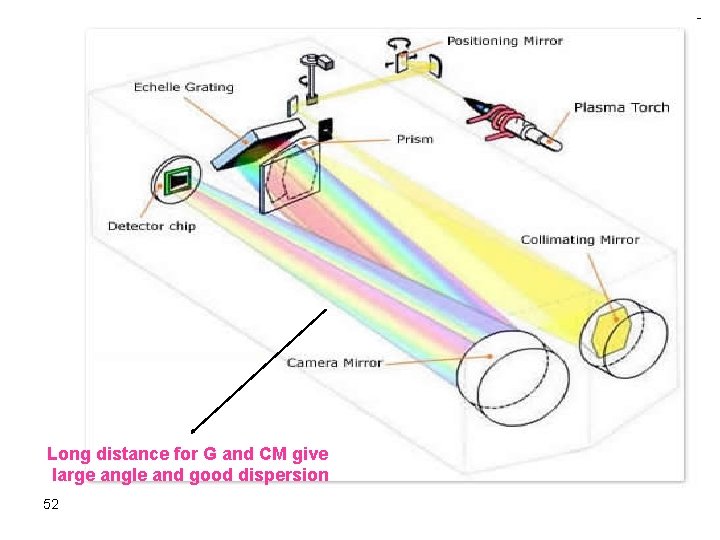
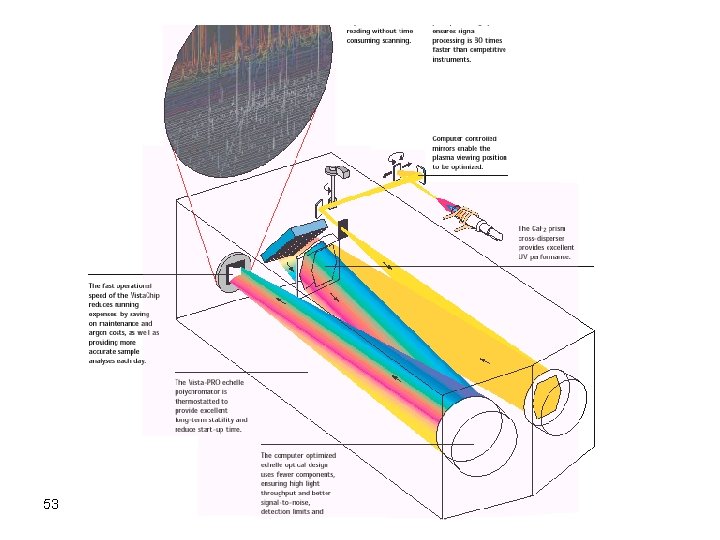
To overcome the previous problem CCD or CID Detector Grating Echele grating (high Res, use the order) 50 Prism to further resolve unresolved lines and the orders of any line Could be used for qualitative analysis

51

Long distance for G and CM give large angle and good dispersion 52

53

3. Fourier transform instruments (FT) Ø Instruments in which the signal is coded will need a decoding mechanism in order to see the signal. Ø FT is a very common technique for decoding time domain spectra. Ø In such instruments, the detector records the change of signal with time, which is practically not useful. Ø However, Fourier transformation of the time domain signal yield a frequency domain spectrum, which is the usual signal, obtained by conventional methods. Ø Instruments that rely on decoding a coded signal is also said to have a multiplex design. 54

Atomic Emission Spectroscopy Lecture 20 55

Applications of Plasma Sources 1. Since plasma sources result in a very large number of emission lines, these sources can be used for both qualitative and quantitative analysis. 2. The signal obtained from plasma sources is stable, has a low noise and background, as well as freedom from interferences. 3. Requires sample preparation similar to AAS 56

4. Plasma sources are usually best suited for operation in the ultraviolet region: Therefore: Ø Elements having emission lines below 180 nm (like B, P, S, N, and C) can only be analyzed under vacuum since air components absorb under 180 nm. Ø Alkali metals are difficult to analyze since their best lines under plasma conditions occur in the visible or near infrared. 5. An analytical emission line can easily be located but will depend on the other elements present since spectral line interferences are encountered in plasma sources due to the very high temperatures used. 57

6. Linear calibration plots are usually obtained. Problem: departure from linearity is observed at high concentrations: due to self absorption as well as other instrumental reasons. Overcome: An internal ( ) ﺧﺼﺎﺋﺼﻪ ﺗﺸﺒﻪ ﺍﻟﻌﻴﻨﺔ ﺍﻟﻤﺠﻬﻮﻟﺔ ﻭﻟﻜﻦ ﻻ ﺗﻤﺘﺺ ﻋﻨﺪ ﻧﻔﺲ ﺍﻟﺨﻂ standard is often used in emission methods to correct for fluctuations in temperature as well as other factors. v The calibration plot in this case is a plot between the concentration of analyte and the ratio of the analyte to internal standard signal. The internal standard: v Is a substance that is added in a constant amount to all samples, blanks, and standards; therefore it must be absent from initial sample matrix. v The internal standard should have very close characteristics (both chemically and physically) to analyte. 58

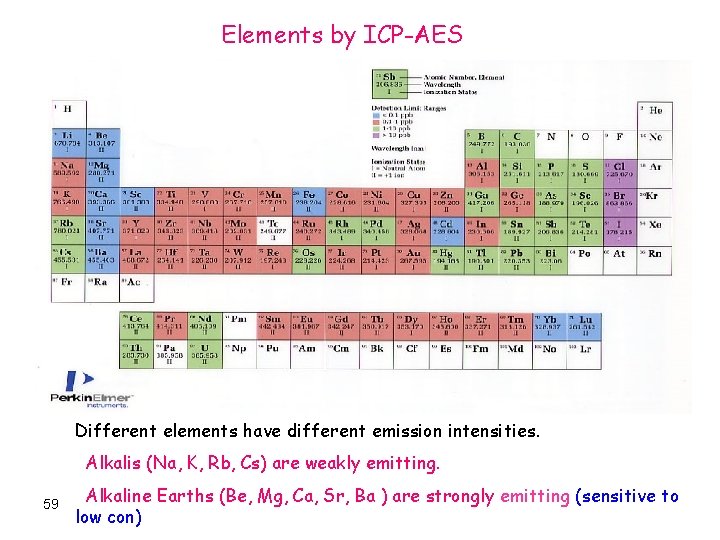
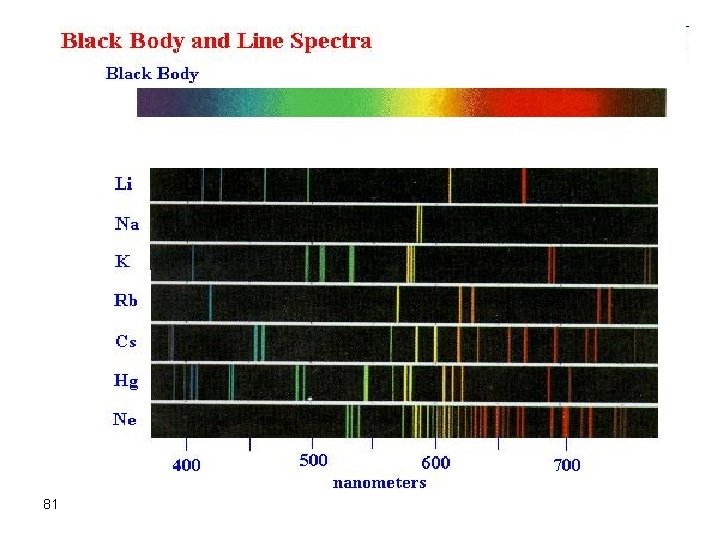
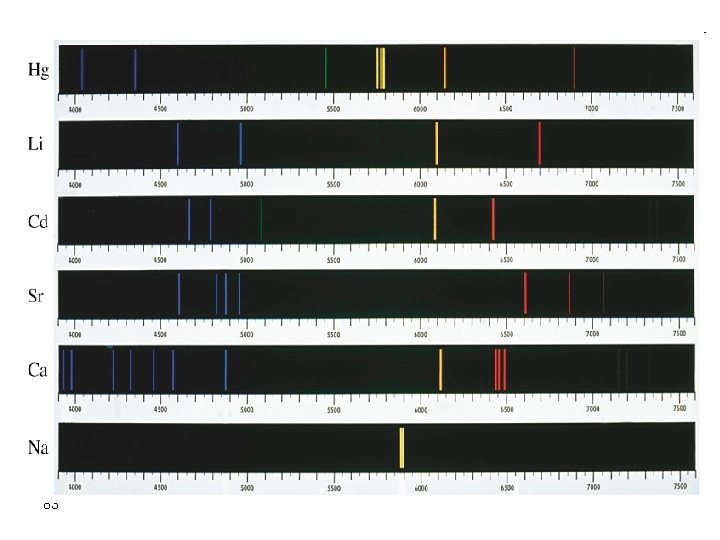
Elements by ICP-AES Different elements have different emission intensities. Alkalis (Na, K, Rb, Cs) are weakly emitting. 59 Alkaline Earths (Be, Mg, Ca, Sr, Ba ) are strongly emitting (sensitive to low con)

background 60

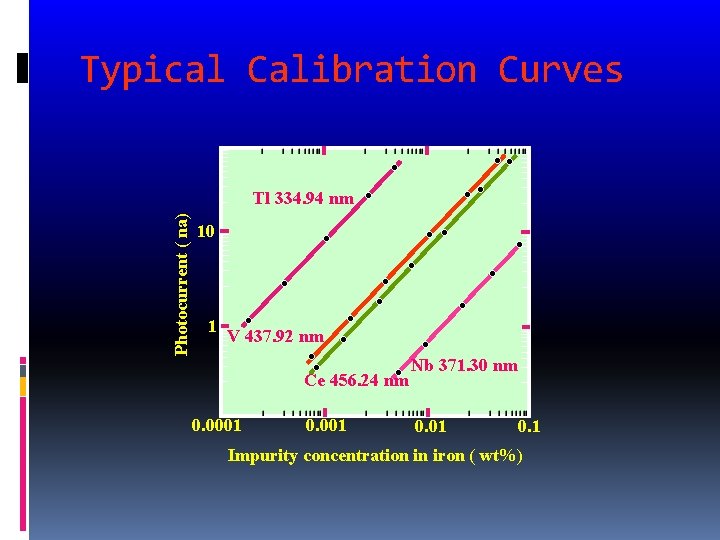
Typical Calibration Curves Photocurrent ( na) Tl 334. 94 nm 10 1 V 437. 92 nm Ce 456. 24 nm 0. 0001 0. 001 Nb 371. 30 nm 0. 01 0. 1 Impurity concentration in iron ( wt%)

INDUCTIVELY COUPLED PLASMA-MASS SPECTROMETRY (ICP-MS) The detector is the MS (used to measure AW for different atoms) - Very sensitive and good for trace analysis. - Plasma produces analyte ions. - Ions are directed to a mass spectrometer. - Ions are separated on the basis of their mass-to-charge ratio. - A very sensitive detector measures ions. - Very low detection limits. 62

Emission Spectroscopy Based on Arcs and Sparks v. Samples are excited in the gap between a pair of electrodes connected to a high potential power supply (200 VDC or 2200 - 4400 VAC). v. The high potential applied forces a discharge between the two electrodes to occur where current passes between the two separated electrodes (temperature rises due to very high resistance). 63

One electrode is graphite and the other is the sample the two electrodes approach to each others 64 Arc

v The very high temperature (4000 -5000 o. C) realized in the vicinity between the two electrodes provide enough energy for atomization and excitation of the samples in this region or when the sample is, or a part of, one of the electrodes. v Arc and spark methods are mainly used as qualitative techniques and can also be used as semiquantitative techniques. 65

Sample Handling and Preparation Ø If the sample is conductive and is of a shape that can be directly used as an electrode (like a piece of metal or coin), that would be the choice for sample introduction in arc and spark techniques. Ø Otherwise, powdered solid samples are mixed with fine graphite and made into a paste. Ø Upon drying, this solid composite can be used as an electrode. Ø The discharge caused by arcs and sparks interacts with the surface of the solid sample creating a plume of very fine particulates and atoms that are excited and emission is collected. Ø The figure below shows some common shapes of graphite electrodes used in arc and spark sources. 66

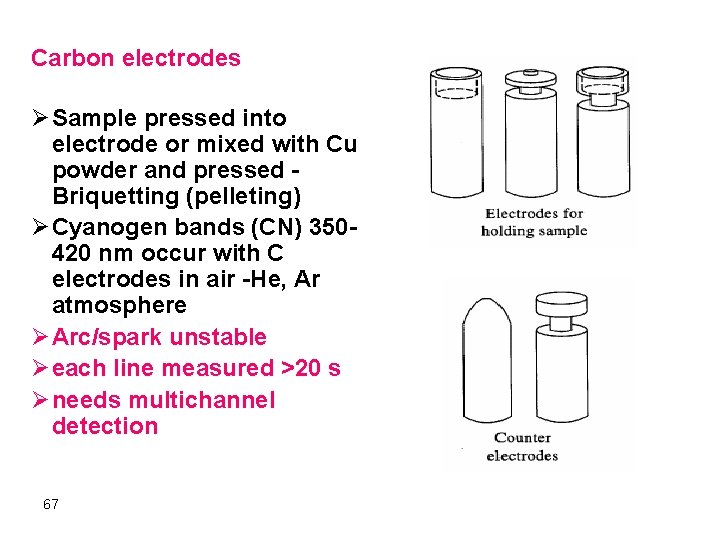
Carbon electrodes Ø Sample pressed into electrode or mixed with Cu powder and pressed Briquetting (pelleting) Ø Cyanogen bands (CN) 350420 nm occur with C electrodes in air -He, Ar atmosphere Ø Arc/spark unstable Ø each line measured >20 s Ø needs multichannel detection 67

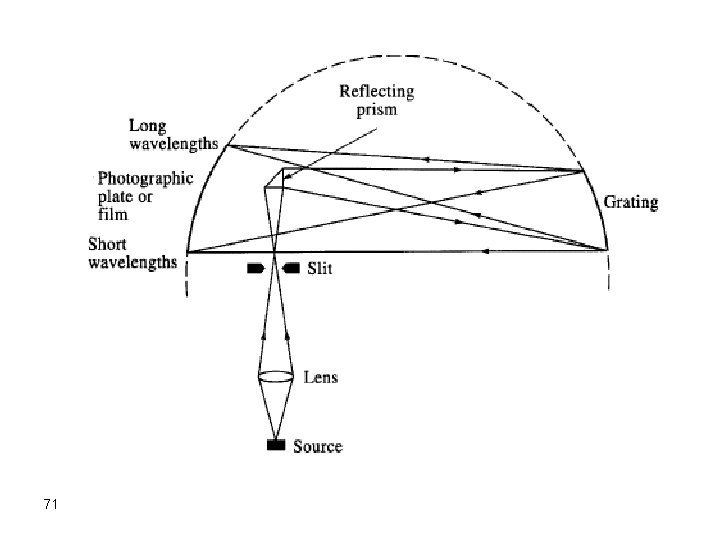
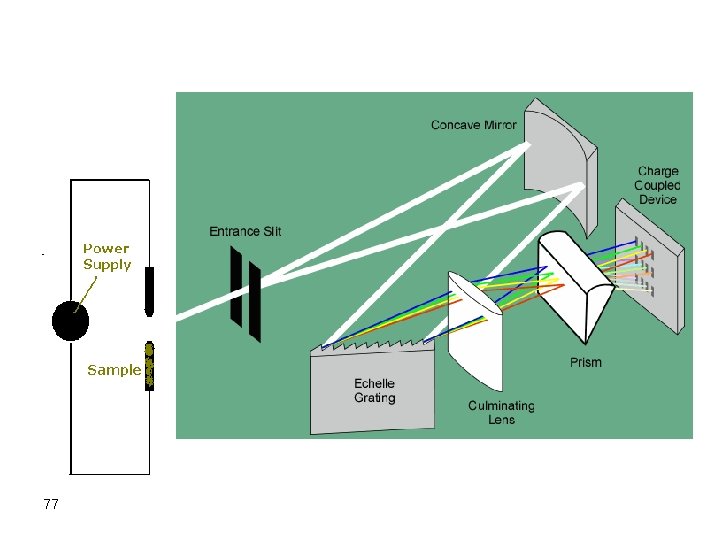
Instruments for Arcs and Sparks v In most cases, emission from atoms in an arc or spark is directed to a monochromator with a long focal length and the diffracted beams are allowed to hit a photographic film. v This typical instrument is called a spectrograph since it uses a photographic film as the detector. 68

Spectrograph Beginning 1930 s (in some old universities) • photographic film detector – Cheap – Long integration times (20 -30 s to obtain stable signal). – Difficult to develop/analyze – Non-linearity of line "darkness“ on the photograph film. 69

Very long monochromator 2 -3 m: For good separation Potential Source Graphite Electrodes Photographic Film 70

71

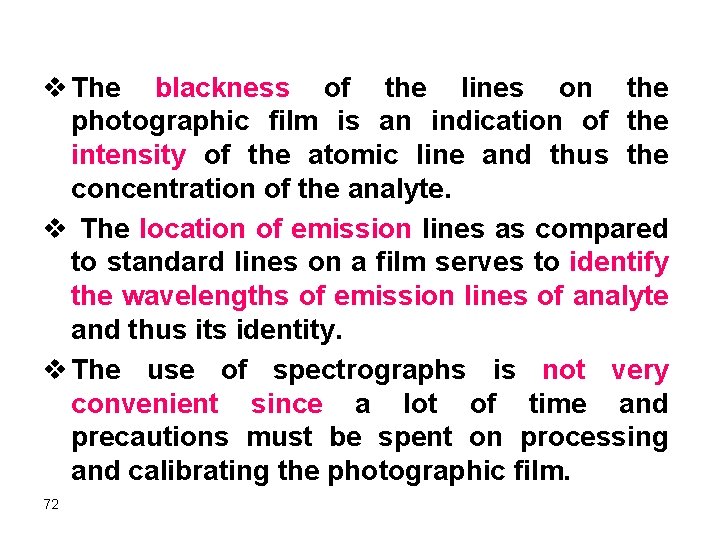
v The blackness of the lines on the photographic film is an indication of the intensity of the atomic line and thus the concentration of the analyte. v The location of emission lines as compared to standard lines on a film serves to identify the wavelengths of emission lines of analyte and thus its identity. v The use of spectrographs is not very convenient since a lot of time and precautions must be spent on processing and calibrating the photographic film. 72

Qualitative analysis: v. Is accomplished by comparison of the wavelengths of some emission lines to standards while the line blackness serves as the tool for semiquantitative analysis. v. Polychromators are also available multichannel arc and spark instruments. as v However, these have fixed slits at certain wavelengths in order to do certain elements and thus they are not versatile. 73

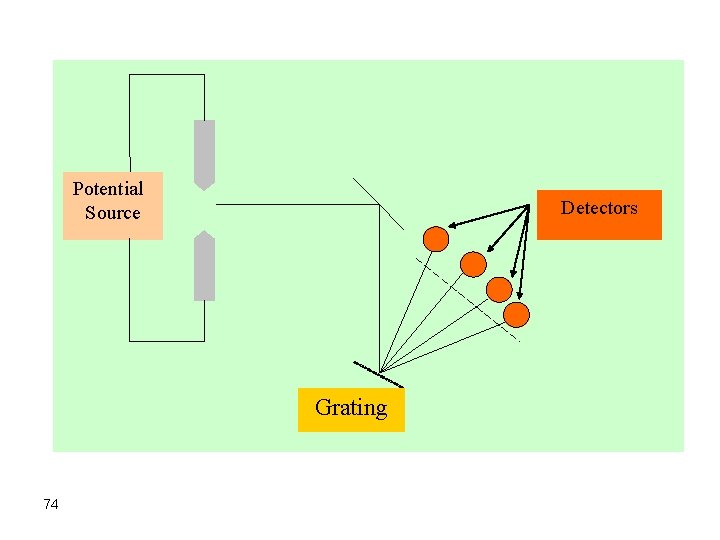
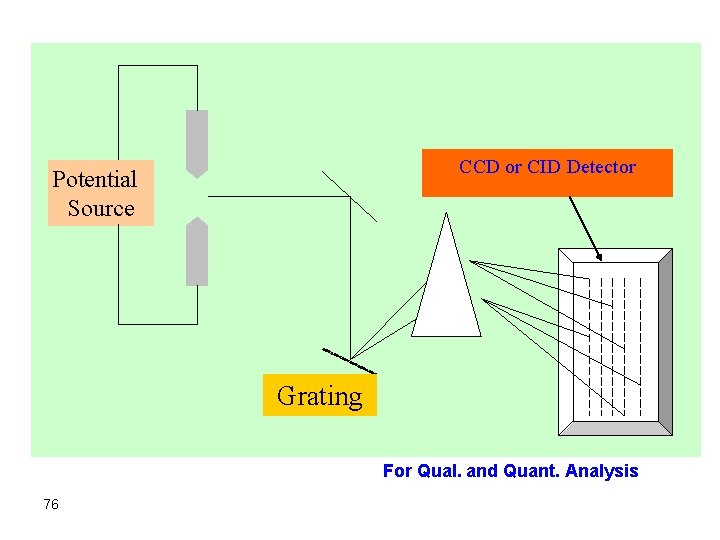
Potential Source Detectors Grating 74

Recently: Ø Arc and spark instruments based on charge injection and charge coupled devices became available. Ø These have extraordinarily high efficiency and performance in terms of: - Easier calibration - Short analysis time - Superior quantitative results. 75

CCD or CID Detector Potential Source Grating For Qual. and Quant. Analysis 76

77

Characteristics of Arc Sources 1. Typical temperatures between 4000 -5000 o. C are high enough to cause atomization and excitation of sample and electrode materials. 2. Usually, cyanogens compounds are formed due to reaction of graphite electrodes with atmospheric nitrogen. Emission bands from cyanogens compounds occur in the region from 350 -420 nm. Disadvantage: Several elements have their most sensitive lines in this same region which limits the technique. Overcome: Use of controlled atmosphere around the arc (using CO 2, Helium, or argon) very much decreases the effect of 78 cyanogens emission.

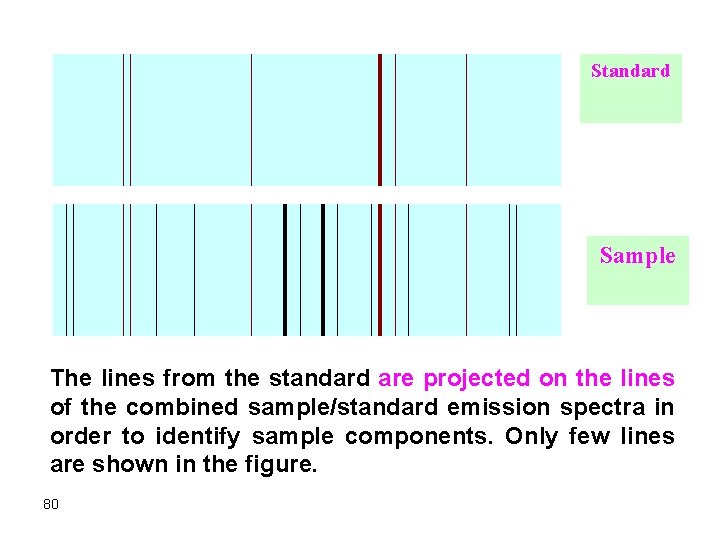
3. The emission signal should be integrated over a minute or so since volatilization and excitation of atoms of different species differ widely. While some species give maximum signal, others may still be in the molecular state. 4. Arc sources are very good for qualitative analysis of elements while only semiquantitative analysis is possible. Ø It is mandatory ﺍﻟﺰﺍﻣﻲ to compare the emission spectrum of a sample with the emission spectrum of a standard. Ø In some cases, a few milligrams of a standard is added to the sample in order to locate the emission lines of the standard and thus identify the emission wavelengths of the different elements in the sample. A comparator ﻣﻘﺎﺭﻧﺔ densitometer ﻣﻘﻴﺎﺱ ﺷﺪﺓ ﺍﻟﻀﻮﺀ : can be 79 used to exactly locate the wavelengths of the standard and the sample components.

Standard Sample The lines from the standard are projected on the lines of the combined sample/standard emission spectra in order to identify sample components. Only few lines are shown in the figure. 80

81

82

83

Why use Carbon in Atomic Spectroscopy? We have previously seen the use of graphite in electrothermal AAS as well as arc and spark AES, even though molecular spectra are real problems in both techniques due to cyanogens compounds absorption and emission. The reasons after graphite common use in atomic spectroscopy can be summarized below: 84

1. 2. 3. 4. 5. 6. 85 It is conductive. It can be obtained in a very pure state. Easily available and cheap. Thermally stable and inert. Carbon has few emission lines. Easily shaped.

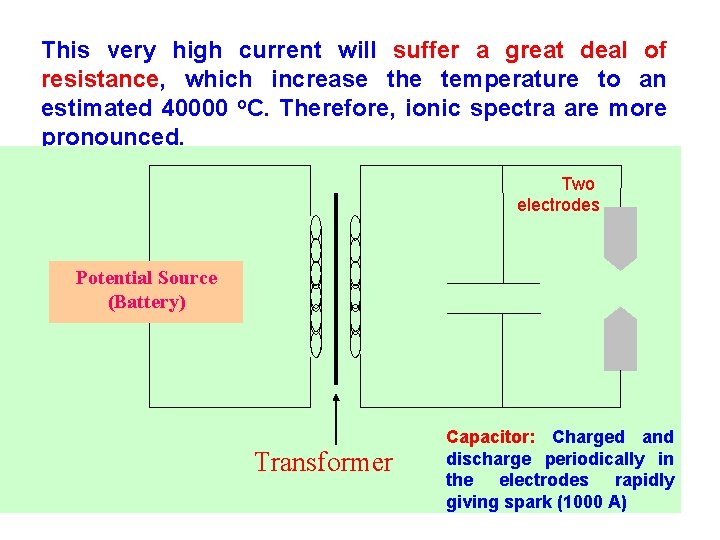
Spark Sources ØMost of the instruments in this category are arc based instruments. Ø Spark based instruments are of the same idea except for a spark source substituting an arc source. Ø The spark source construction: An AC potential in the order of 10 -50 KV is discharged through a capacitor which is charged and discharged through the graphite electrodes about 120 times/s; resulting in a discharge current of about 1000 A. 86

This very high current will suffer a great deal of resistance, which increase the temperature to an estimated 40000 o. C. Therefore, ionic spectra are more pronounced. Two electrodes Potential Source (Battery) Transformer 87 Capacitor: Charged and discharge periodically in the electrodes rapidly giving spark (1000 A)