Atomic Calculations and Mass Spectrometry Quantum Orbital Video

Atomic Calculations and Mass Spectrometry Quantum Orbital Video
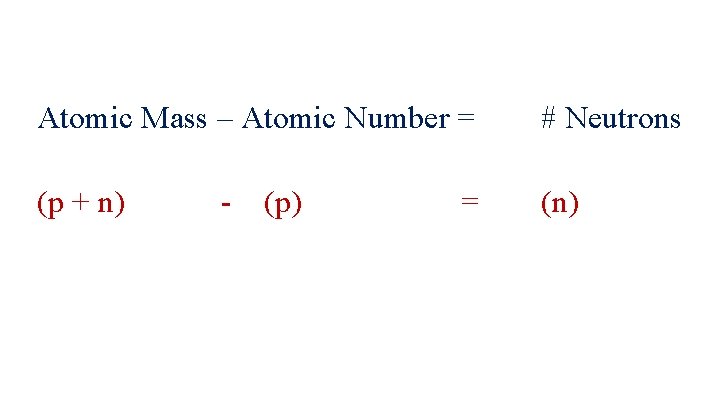
Atomic Mass – Atomic Number = # Neutrons (p + n) (n) - (p) =

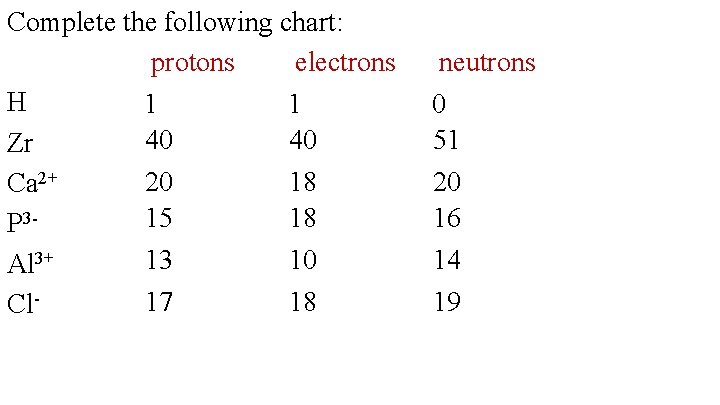
Complete the following chart: protons electrons H 1 1 40 40 Zr Ca 2+ P 3 Al 3+ Cl- neutrons 0 51 20 15 13 18 18 10 20 16 14 17 18 19
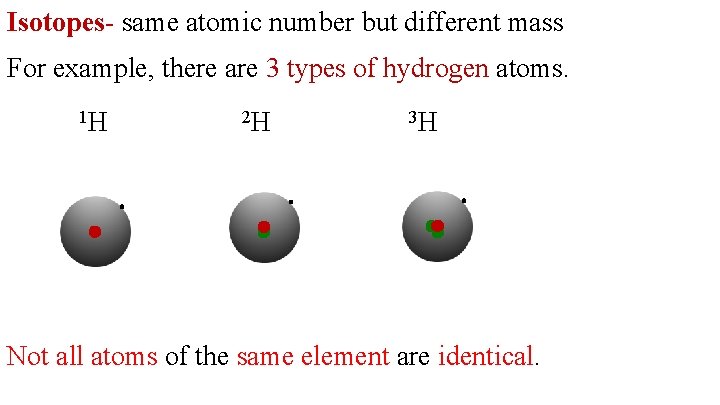
Isotopes- same atomic number but different mass For example, there are 3 types of hydrogen atoms. 1 H 2 H 3 H Not all atoms of the same element are identical.

Mass Spectrometers Are used to determine the abundance and mass of the isotopes of elements

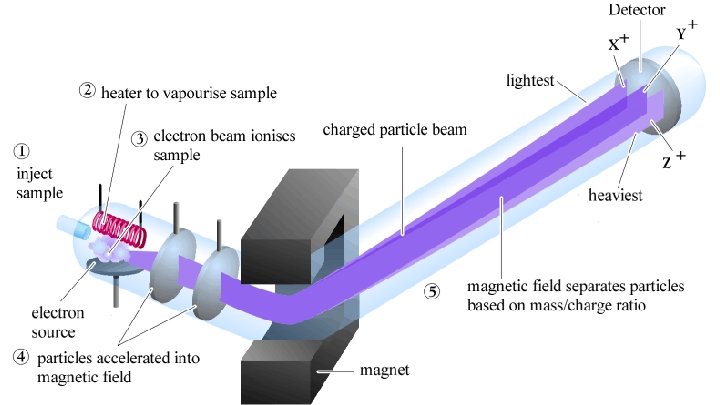
Mass Spectrometers A device known as a mass spectrometer can be used to determine the relative abundance and the mass of the isotopes of elements. In the graph the isotopes of a sample of the element zirconium can be seen in their relative abundances.
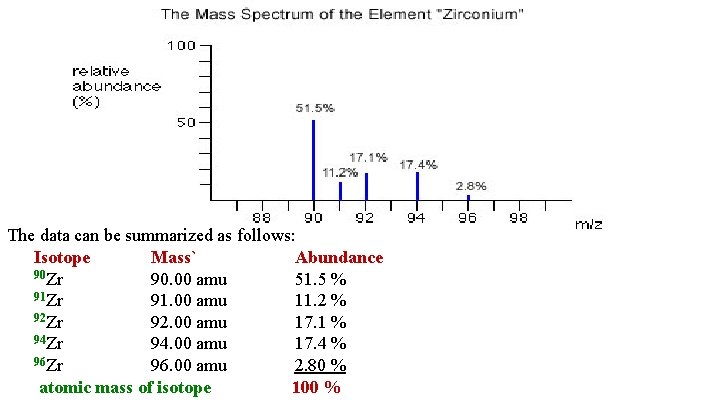
The data can be summarized as follows: Isotope Mass` Abundance 90 Zr 90. 00 amu 51. 5 % 91 Zr 91. 00 amu 11. 2 % 92 Zr 92. 00 amu 17. 1 % 94 Zr 94. 00 amu 17. 4 % 96 Zr 96. 00 amu 2. 80 % atomic mass of isotope 100 %
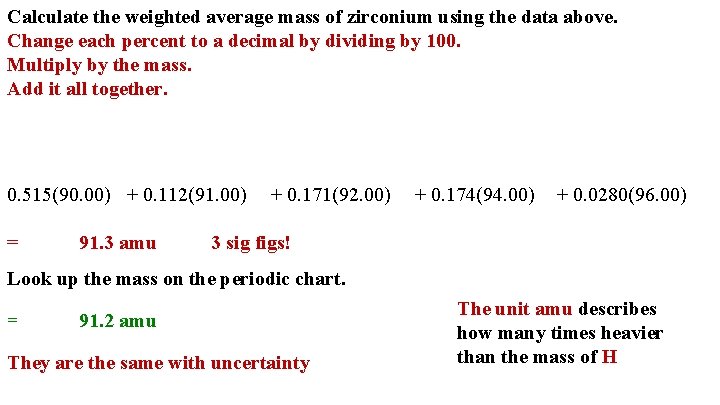
Calculate the weighted average mass of zirconium using the data above. Change each percent to a decimal by dividing by 100. Multiply by the mass. Add it all together. 0. 515(90. 00) + 0. 112(91. 00) = 91. 3 amu + 0. 171(92. 00) + 0. 174(94. 00) + 0. 0280(96. 00) 3 sig figs! Look up the mass on the periodic chart. = 91. 2 amu They are the same with uncertainty The unit amu describes how many times heavier than the mass of H
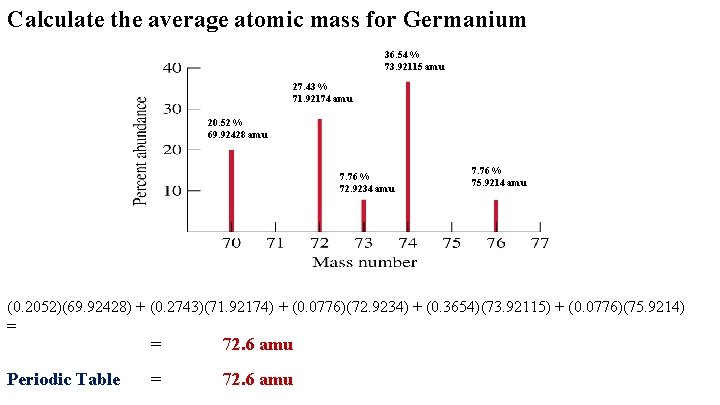
Calculate the average atomic mass for Germanium 36. 54 % 73. 92115 amu 27. 43 % 71. 92174 amu 20. 52 % 69. 92428 amu 7. 76 % 72. 9234 amu 7. 76 % 75. 9214 amu (0. 2052)(69. 92428) + (0. 2743)(71. 92174) + (0. 0776)(72. 9234) + (0. 3654)(73. 92115) + (0. 0776)(75. 9214) = Periodic Table = 72. 6 amu

Quantum orbitals
- Slides: 13