Atomic Absorption Terry A Ring Chemical Engineering University

Atomic Absorption Terry A. Ring Chemical Engineering University of Utah
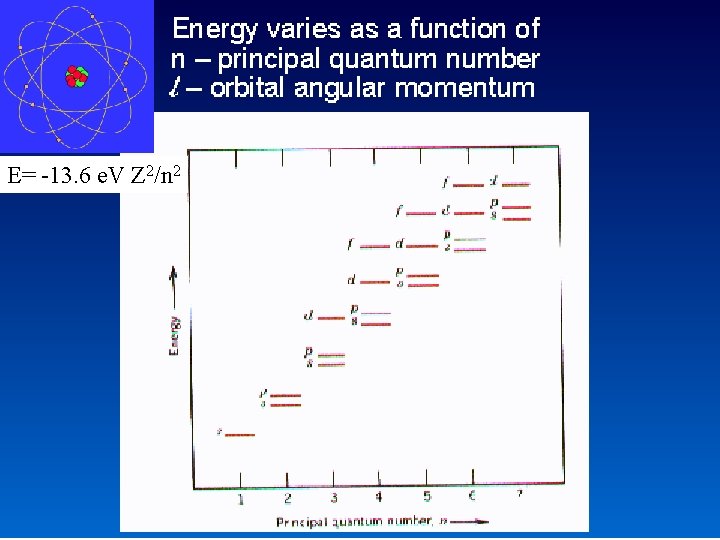
E= -13. 6 e. V Z 2/n 2
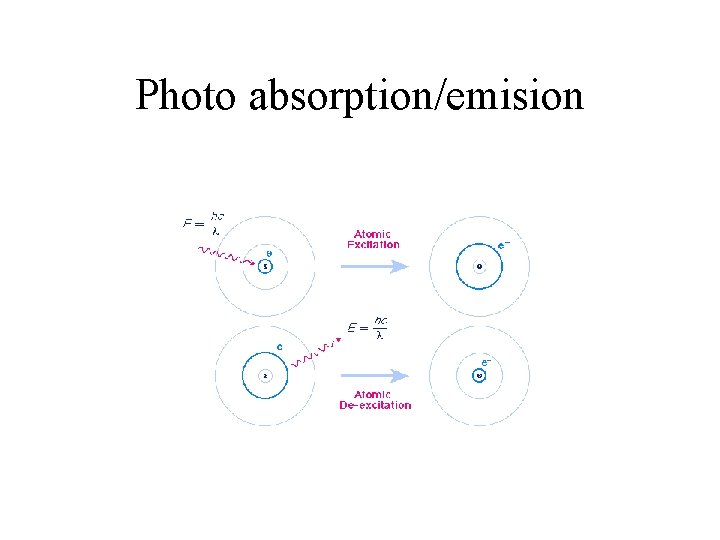
Photo absorption/emision
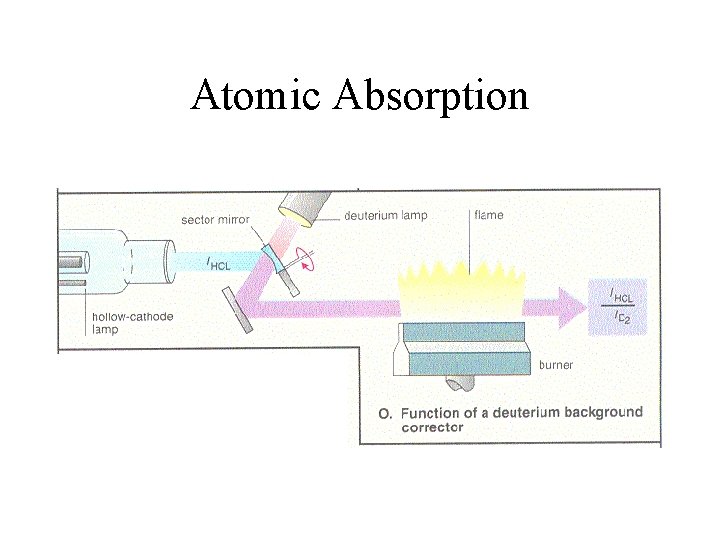
Atomic Absorption

Lamps are Special • Cathode with receptacle for material • Vapor of Material to be analyzed • Vapor Excited by plasma • Light of particular wavelength
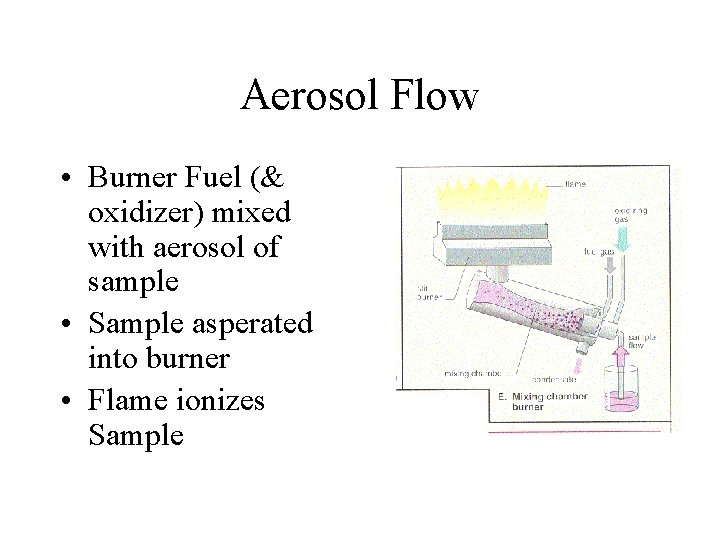
Aerosol Flow • Burner Fuel (& oxidizer) mixed with aerosol of sample • Sample asperated into burner • Flame ionizes Sample
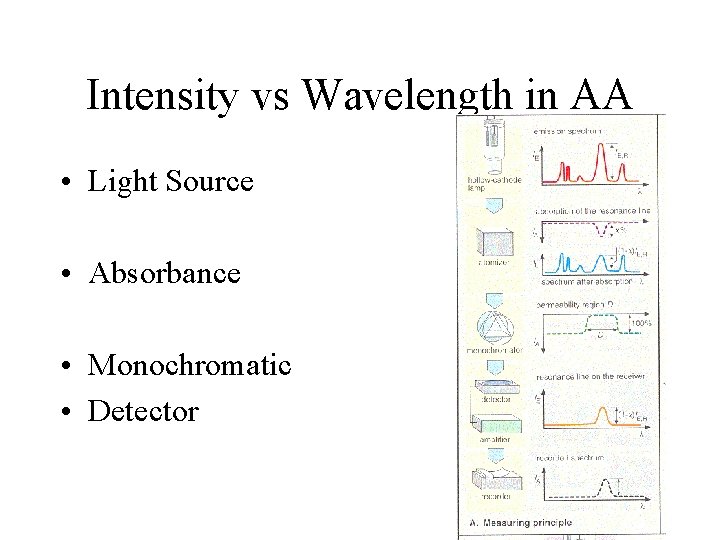
Intensity vs Wavelength in AA • Light Source • Absorbance • Monochromatic • Detector
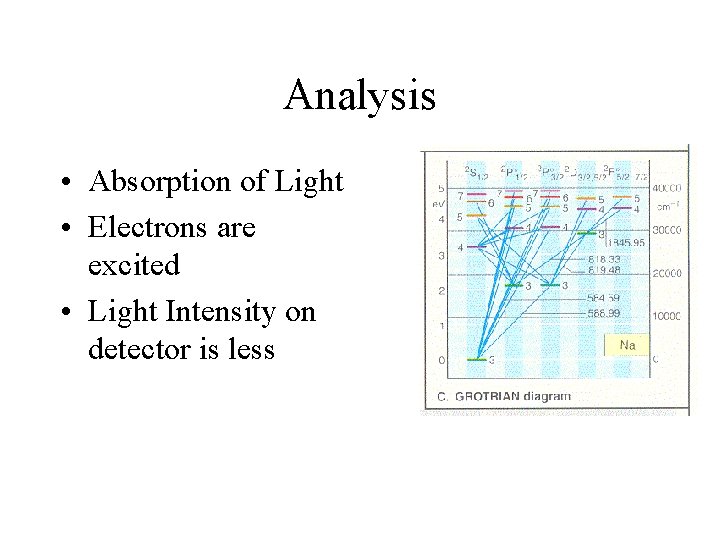
Analysis • Absorption of Light • Electrons are excited • Light Intensity on detector is less
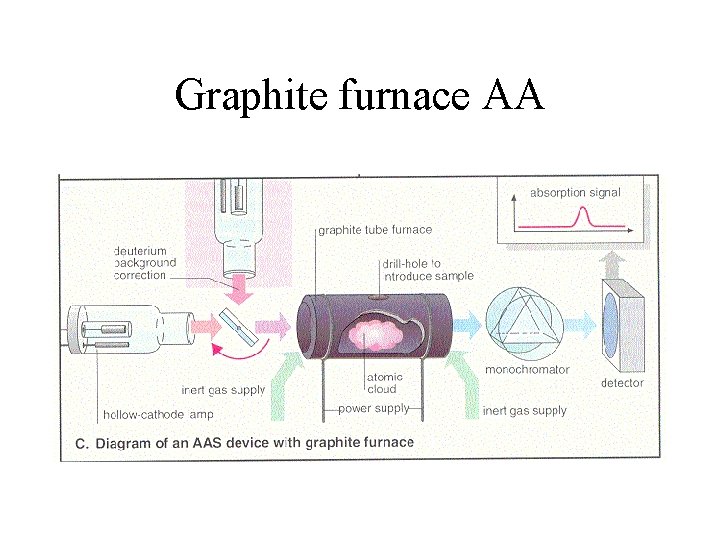
Graphite furnace AA

Other AA’s • • • Flame Spark Arc Plasma Laser X-ray
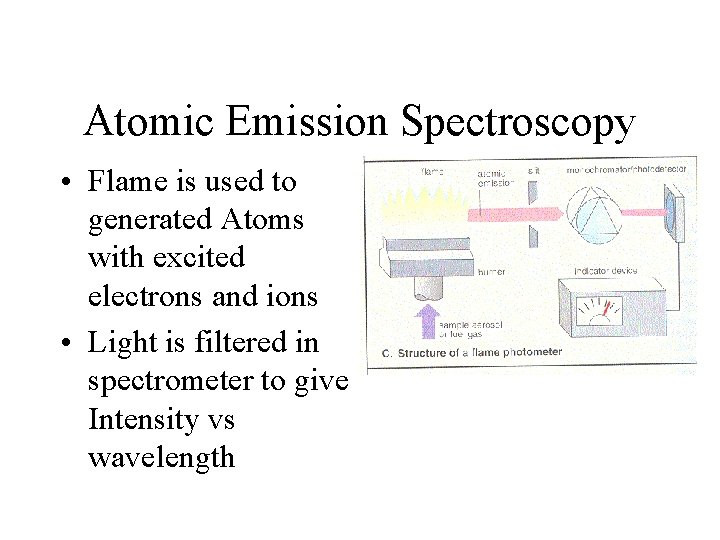
Atomic Emission Spectroscopy • Flame is used to generated Atoms with excited electrons and ions • Light is filtered in spectrometer to give Intensity vs wavelength
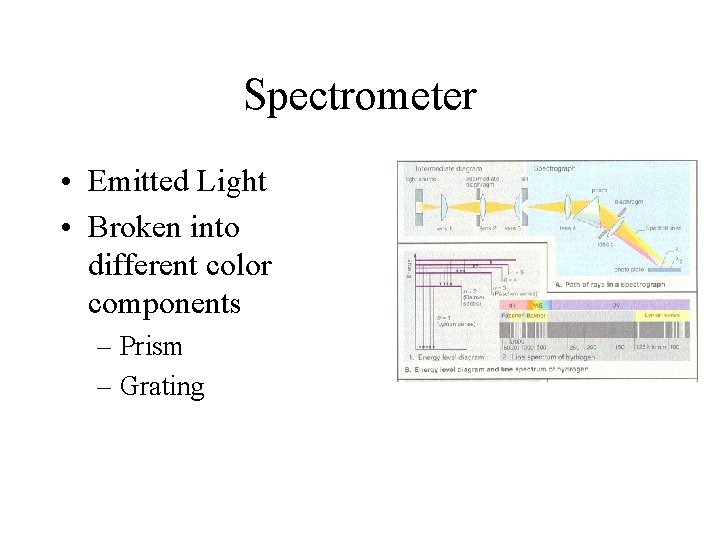
Spectrometer • Emitted Light • Broken into different color components – Prism – Grating
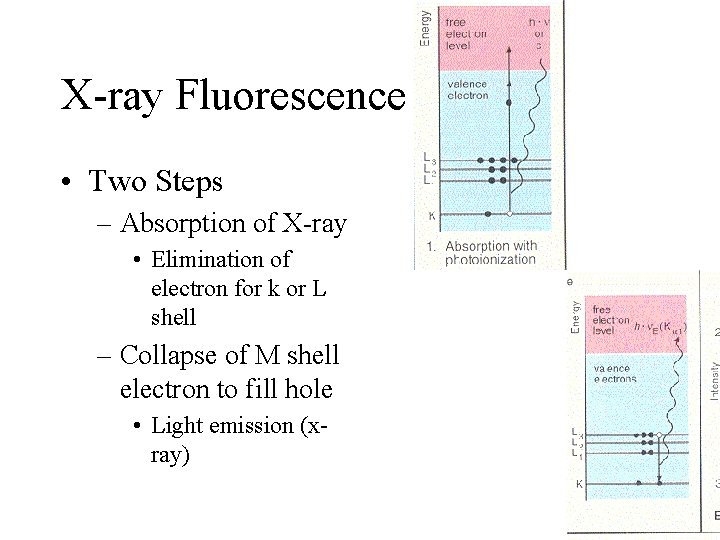
X-ray Fluorescence • Two Steps – Absorption of X-ray • Elimination of electron for k or L shell – Collapse of M shell electron to fill hole • Light emission (xray)
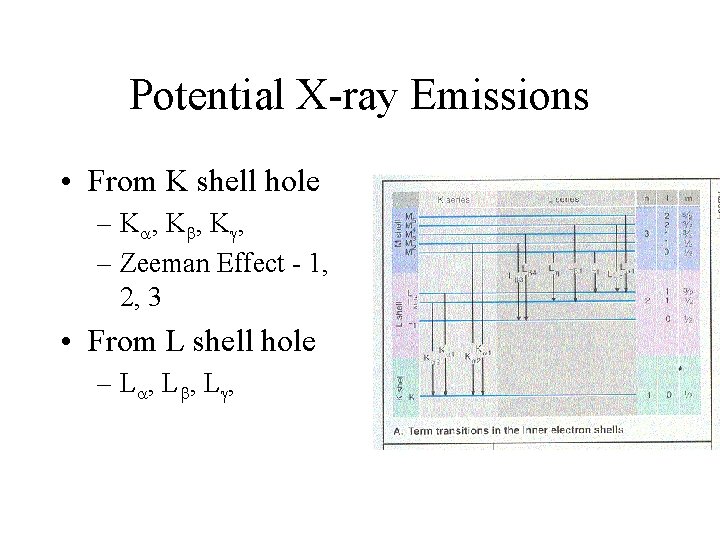
Potential X-ray Emissions • From K shell hole – K , K , – Zeeman Effect - 1, 2, 3 • From L shell hole – L , L ,
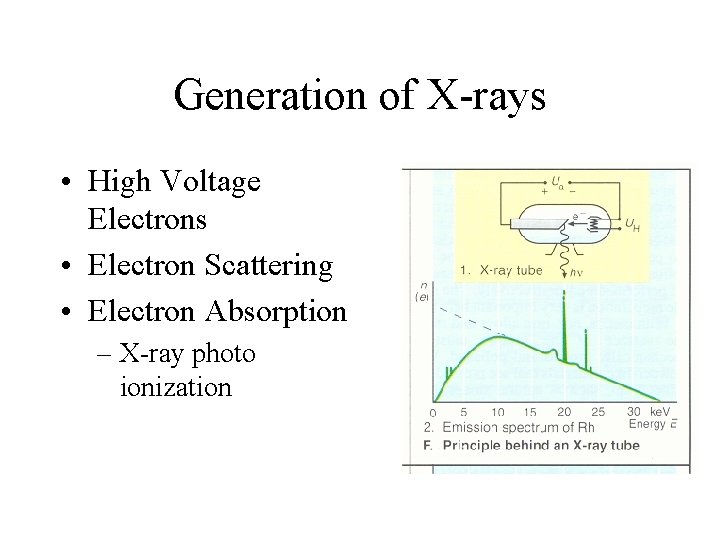
Generation of X-rays • High Voltage Electrons • Electron Scattering • Electron Absorption – X-ray photo ionization
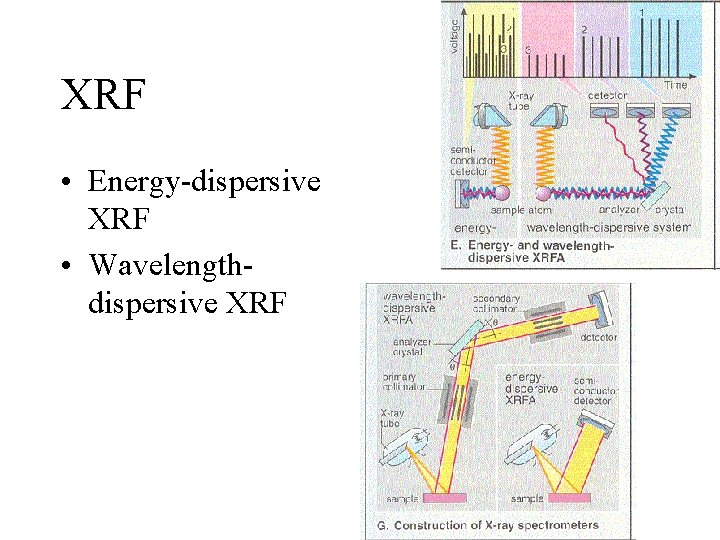
XRF • Energy-dispersive XRF • Wavelengthdispersive XRF
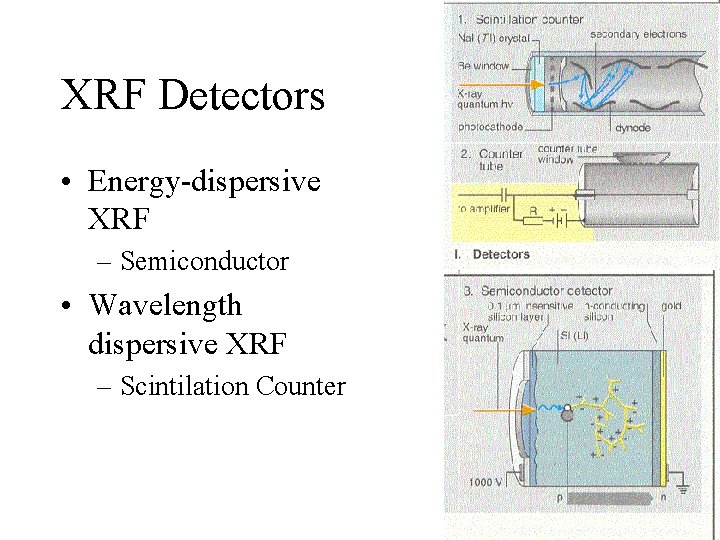
XRF Detectors • Energy-dispersive XRF – Semiconductor • Wavelength dispersive XRF – Scintilation Counter
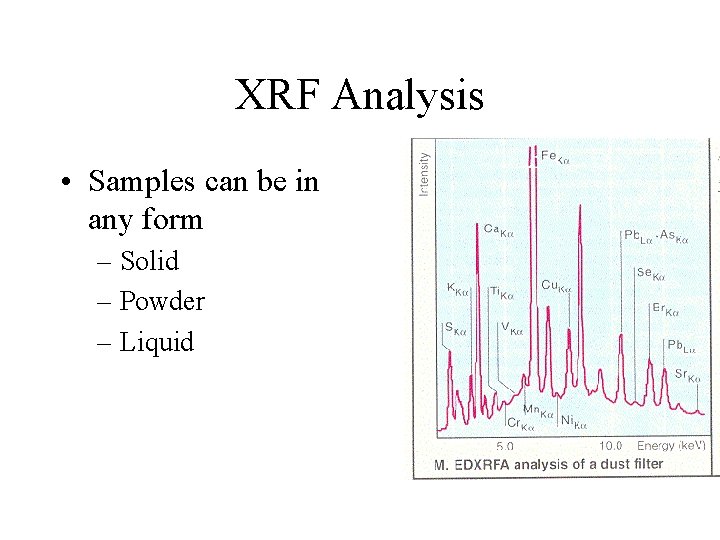
XRF Analysis • Samples can be in any form – Solid – Powder – Liquid
- Slides: 18