Atom smallest particle of an element that retains
- Slides: 10

• Atom – smallest particle of an element that retains the characteristics of that element. • Element – the most simple chemical substance • Arranged in the periodic table – Columns and rows – Each element is identified via 1 letter or 2 letter abbreviation Atoms

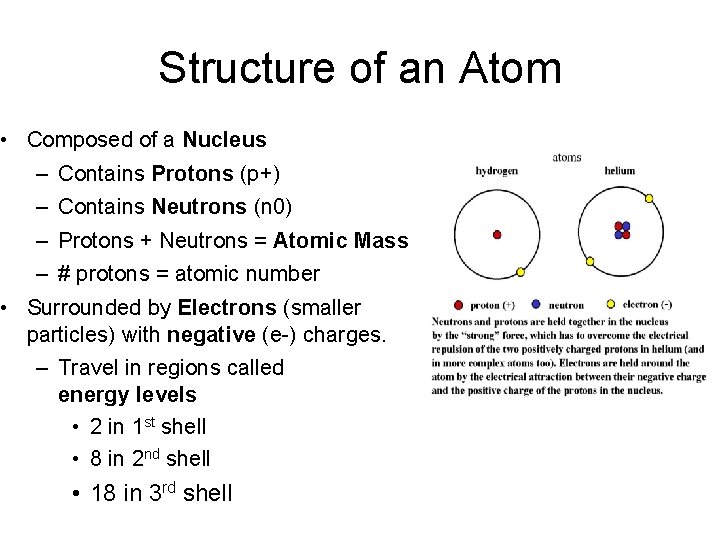
Structure of an Atom • Composed of a Nucleus – Contains Protons (p+) – Contains Neutrons (n 0) – Protons + Neutrons = Atomic Mass – # protons = atomic number • Surrounded by Electrons (smaller particles) with negative (e-) charges. – Travel in regions called energy levels • 2 in 1 st shell • 8 in 2 nd shell • 18 in 3 rd shell

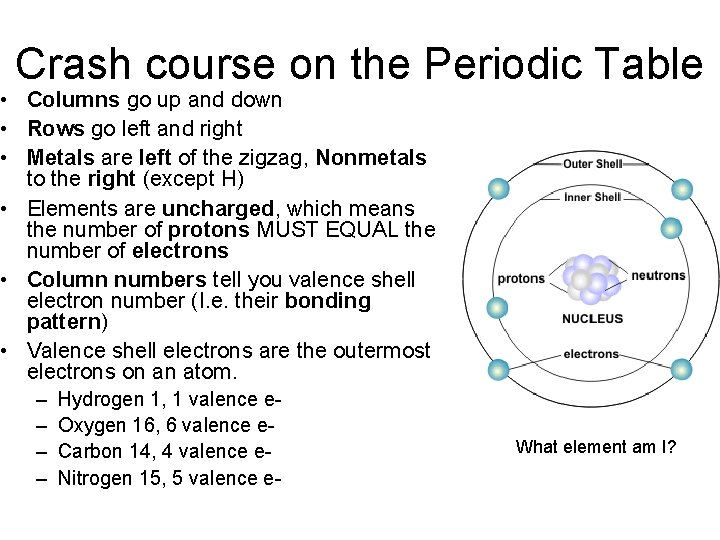
Crash course on the Periodic Table • Columns go up and down • Rows go left and right • Metals are left of the zigzag, Nonmetals to the right (except H) • Elements are uncharged, which means the number of protons MUST EQUAL the number of electrons • Column numbers tell you valence shell electron number (I. e. their bonding pattern) • Valence shell electrons are the outermost electrons on an atom. – – Hydrogen 1, 1 valence e. Oxygen 16, 6 valence e. Carbon 14, 4 valence e. Nitrogen 15, 5 valence e- What element am I? ___________atom am I?

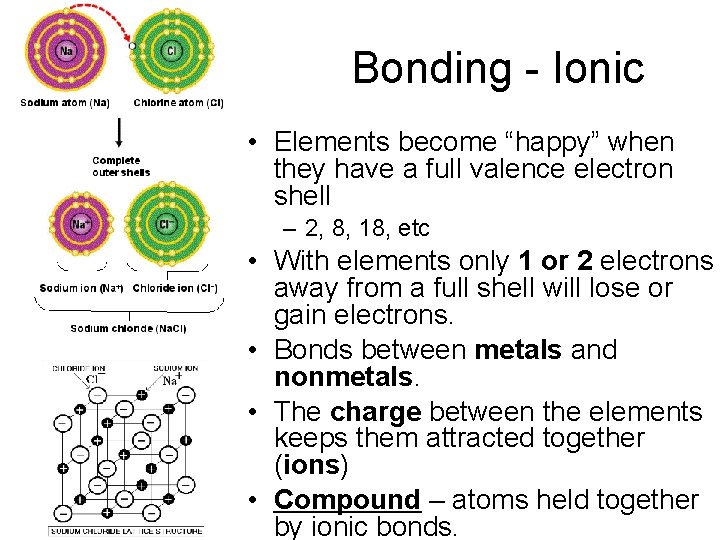
Bonding - Ionic • Elements become “happy” when they have a full valence electron shell – 2, 8, 18, etc • With elements only 1 or 2 electrons away from a full shell will lose or gain electrons. • Bonds between metals and nonmetals. • The charge between the elements keeps them attracted together (ions) • Compound – atoms held together by ionic bonds.

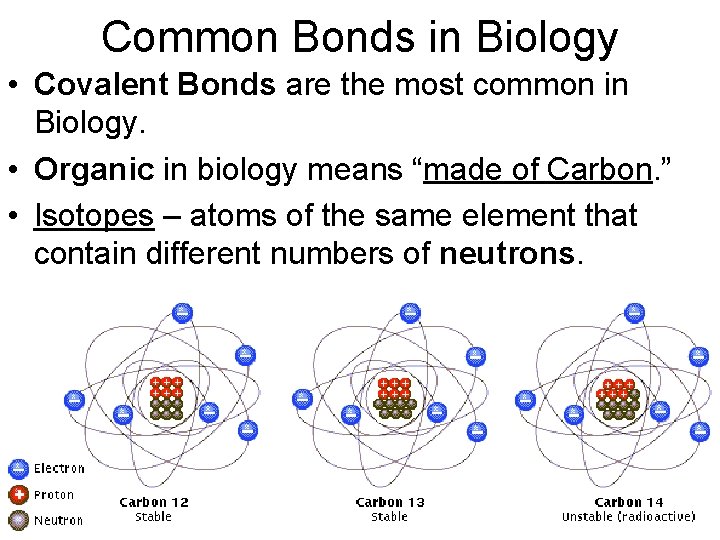
Common Bonds in Biology • Covalent Bonds are the most common in Biology. • Organic in biology means “made of Carbon. ” • Isotopes – atoms of the same element that contain different numbers of neutrons.

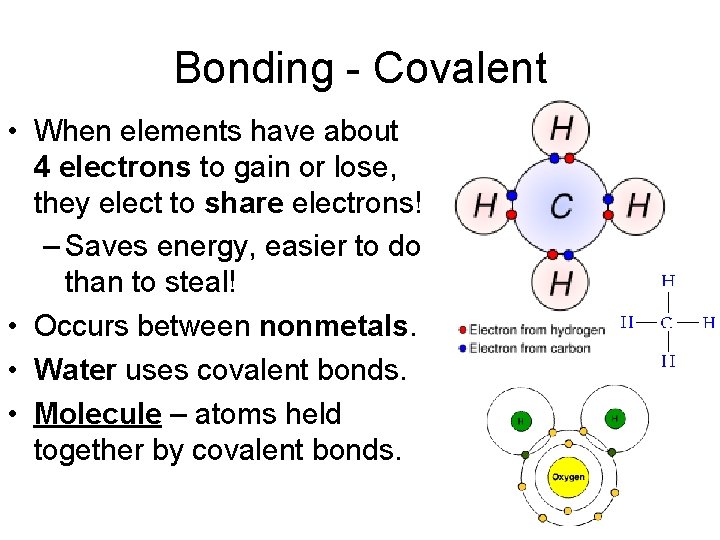
Bonding - Covalent • When elements have about 4 electrons to gain or lose, they elect to share electrons! – Saves energy, easier to do than to steal! • Occurs between nonmetals. • Water uses covalent bonds. • Molecule – atoms held together by covalent bonds.

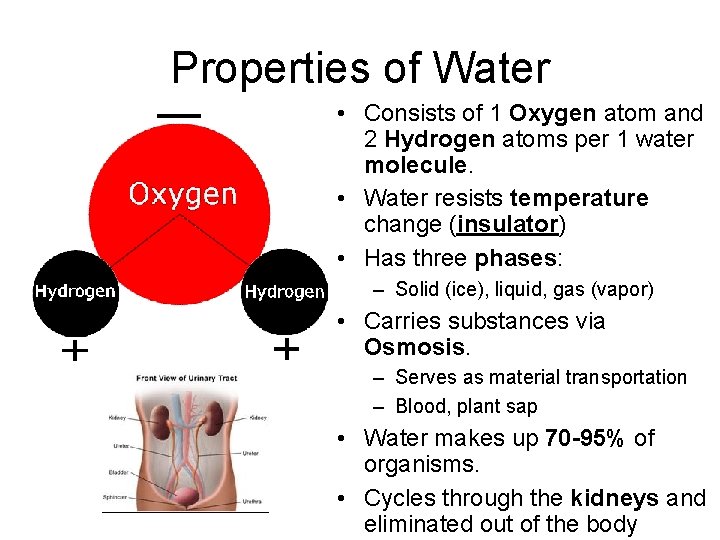
Properties of Water • Consists of 1 Oxygen atom and 2 Hydrogen atoms per 1 water molecule. • Water resists temperature change (insulator) • Has three phases: – Solid (ice), liquid, gas (vapor) • Carries substances via Osmosis. – Serves as material transportation – Blood, plant sap • Water makes up 70 -95% of organisms. • Cycles through the kidneys and eliminated out of the body

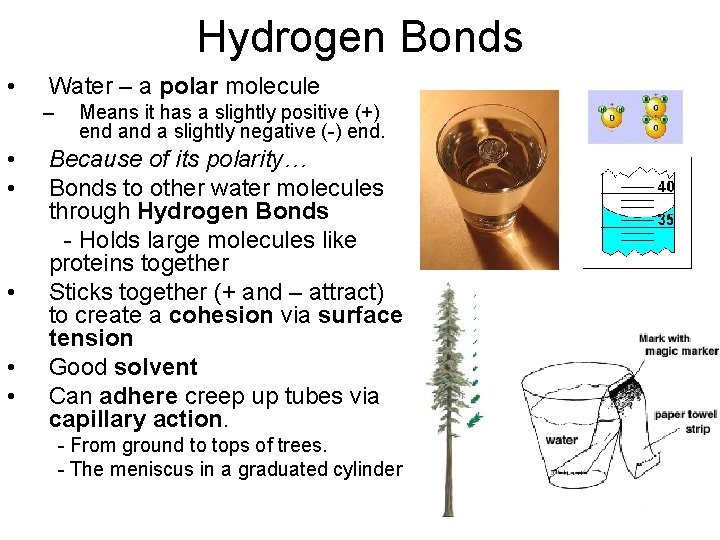
Hydrogen Bonds • Water – a polar molecule – • • • Means it has a slightly positive (+) end a slightly negative (-) end. Because of its polarity… Bonds to other water molecules through Hydrogen Bonds - Holds large molecules like proteins together Sticks together (+ and – attract) to create a cohesion via surface tension Good solvent Can adhere creep up tubes via capillary action. - From ground to tops of trees. - The meniscus in a graduated cylinder

Mixtures • A mixture is a combination of substances where both substances keep their original properties. – Physically mixed, not chemically mixed. • Solution – one or more substances (solutes) are distributed evenly in another substance (solvent) (water) – The more solute in a solvent, the higher the concentration. – Organisms need to maintain a certain concentration for life processes, called homeostasis. • Suspensions – mixtures of water and non dissolved particles – Example: Blood

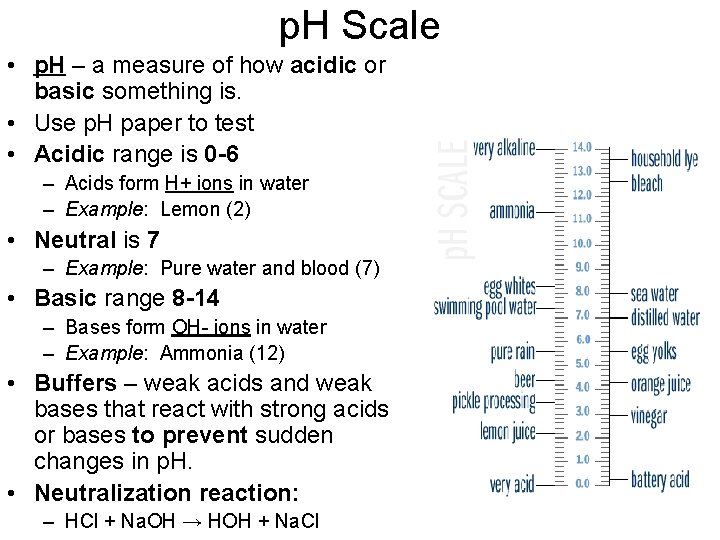
p. H Scale • p. H – a measure of how acidic or basic something is. • Use p. H paper to test • Acidic range is 0 -6 – Acids form H+ ions in water – Example: Lemon (2) • Neutral is 7 – Example: Pure water and blood (7) • Basic range 8 -14 – Bases form OH- ions in water – Example: Ammonia (12) • Buffers – weak acids and weak bases that react with strong acids or bases to prevent sudden changes in p. H. • Neutralization reaction: – HCl + Na. OH → HOH + Na. Cl