ATMOSPHERIC CHEMISTRY OPTION 1 B INTRODUCTION In this

ATMOSPHERIC CHEMISTRY OPTION 1 B

INTRODUCTION In this section we will discuss: • The ozone layer • The effects of CFCs on the ozone layer • The build up of greenhouse gases
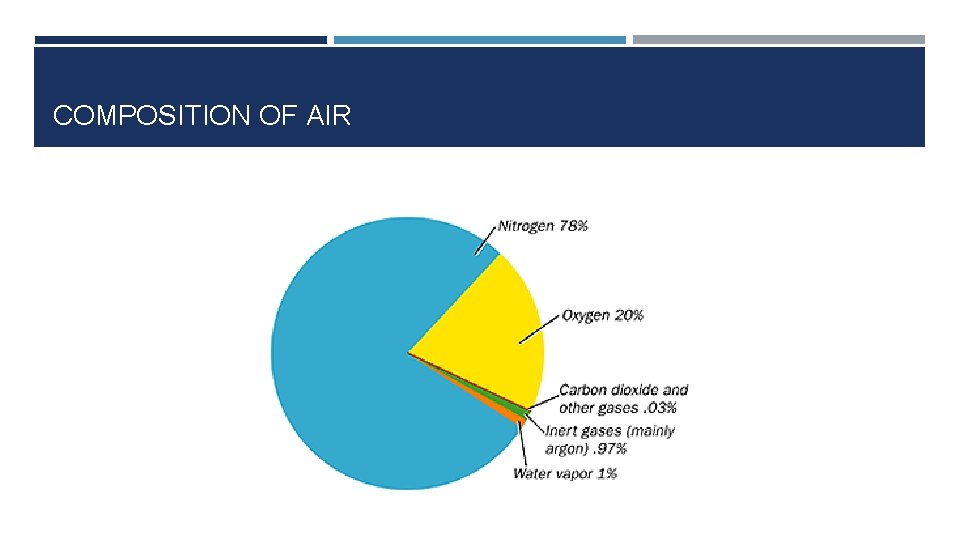
COMPOSITION OF AIR

OXYGEN – THE REACTIVE GAS • Oxygen is the most reactive gas in air Uses of oxygen: • • • Hospitals Steel industry (55% of all oxygen) Rocket fuels Welding Pumped into lakes to combat pollution
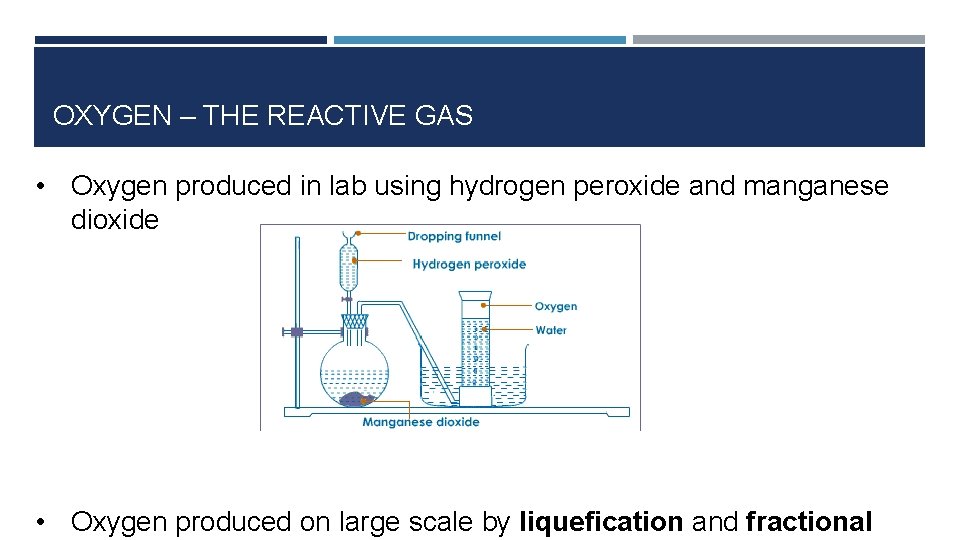
OXYGEN – THE REACTIVE GAS • Oxygen produced in lab using hydrogen peroxide and manganese dioxide • Oxygen produced on large scale by liquefication and fractional
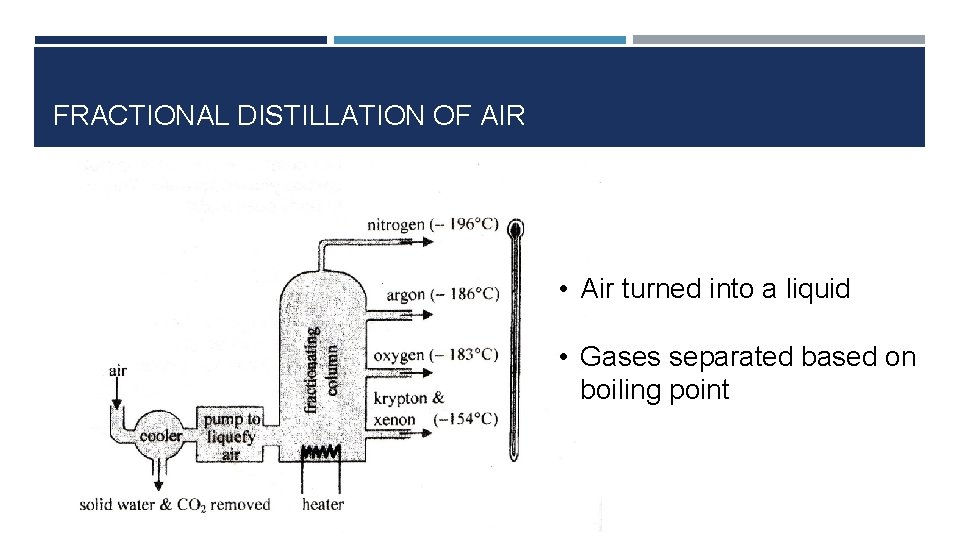
FRACTIONAL DISTILLATION OF AIR • Air turned into a liquid • Gases separated based on boiling point
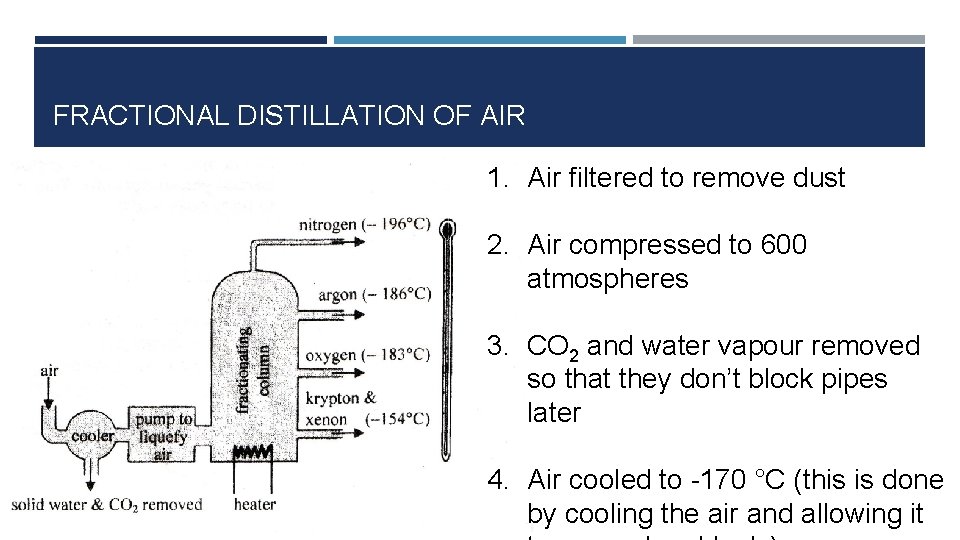
FRACTIONAL DISTILLATION OF AIR 1. Air filtered to remove dust 2. Air compressed to 600 atmospheres 3. CO 2 and water vapour removed so that they don’t block pipes later 4. Air cooled to -170 °C (this is done by cooling the air and allowing it
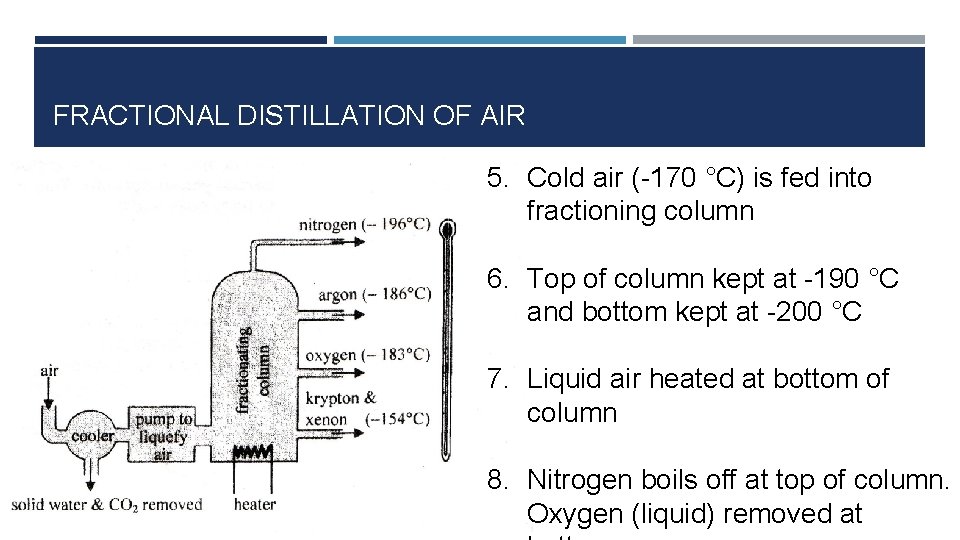
FRACTIONAL DISTILLATION OF AIR 5. Cold air (-170 °C) is fed into fractioning column 6. Top of column kept at -190 °C and bottom kept at -200 °C 7. Liquid air heated at bottom of column 8. Nitrogen boils off at top of column. Oxygen (liquid) removed at

NITROGEN – THE UNREACTIVE GAS • Colourless, odourless, tasteless gas • Unreactive Uses of nitrogen: • • • Bags of crisps filled with nitrogen (to prevent oxidation of oil) Used in transport of flammable chemicals Manufacture of ammonia Liquid nitrogen used to quickly freeze food Liquid nitrogen used to remove warts

LIQUID NITROGEN http: //youtu. be/Kl. Al 5 RHAAns

NITROGEN – THE UNREACTIVE GAS Why is nitrogen unreactive? • Large amount of energy required to break triple bond Is nitrogen polar or non-polar? • It is a non-polar molecule so only slightly soluble in water
NITROGEN – THE UNREACTIVE GAS • Nitrogen = 78% of air • Essential for plant growth • But atmospheric nitrogen is unreactive and cannot be used by plants Nitrogen fixation = the conversion of atmospheric nitrogen to compounds which can be used by plants
NITROGEN – THE UNREACTIVE GAS In nature, nitrogen fixation takes place in two ways: 1. Electricity during a thunderstorm causes oxygen and nitrogen to react to produce nitrogen monoxide N 2 + O 2 • The nitrogen monoxide reacts with oxygen to form nitrogen dioxide: 2 NO + • 2 NO O 2 2 NO 2 The nitrogen dioxide dissolves in rainwater to produce nitrous acid and nitric acid 2 NO 2 + H 2 O HNO 2 + HNO 3

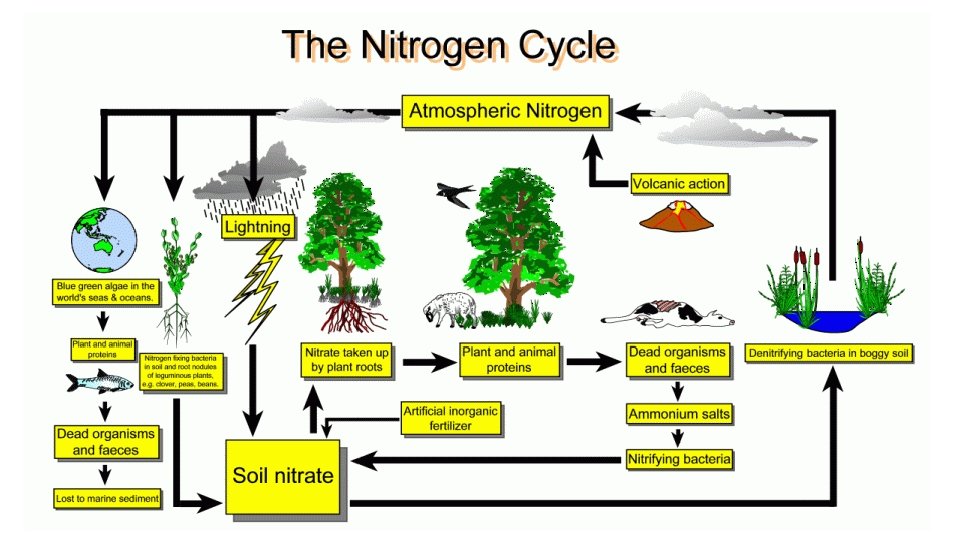
NITROGEN – THE UNREACTIVE GAS In nature, nitrogen fixation takes place in two ways: 2. Atmospheric nitrogen converted to nitrogen plants can use by nitrogen-fixing bacteria • Legumes (peas, beans, clover etc. ) have swellings in their roots containing rhizobium bacteria • Not yet fully understood how rhizobium convert N 2 into nitrate ions (NO 3 -)
NITROGEN – THE UNREACTIVE GAS

INORGANIC CARBON COMPOUNDS A few carbon compounds are not classified as organic: • Carbon dioxide • Carbon monoxide • Carbonate compounds • Hydrogencarbonate compounds • Carbide compounds
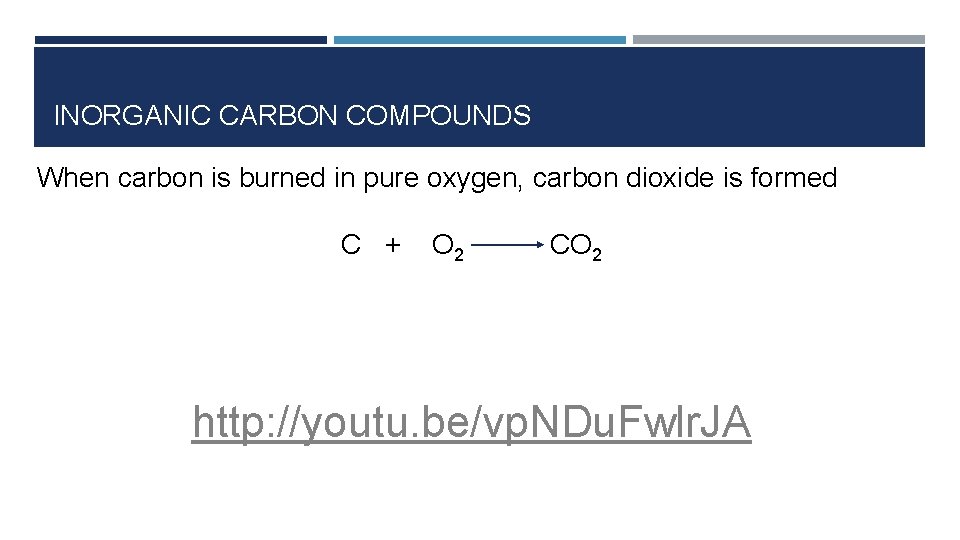
INORGANIC CARBON COMPOUNDS When carbon is burned in pure oxygen, carbon dioxide is formed C + O 2 CO 2 http: //youtu. be/vp. NDu. Fwlr. JA
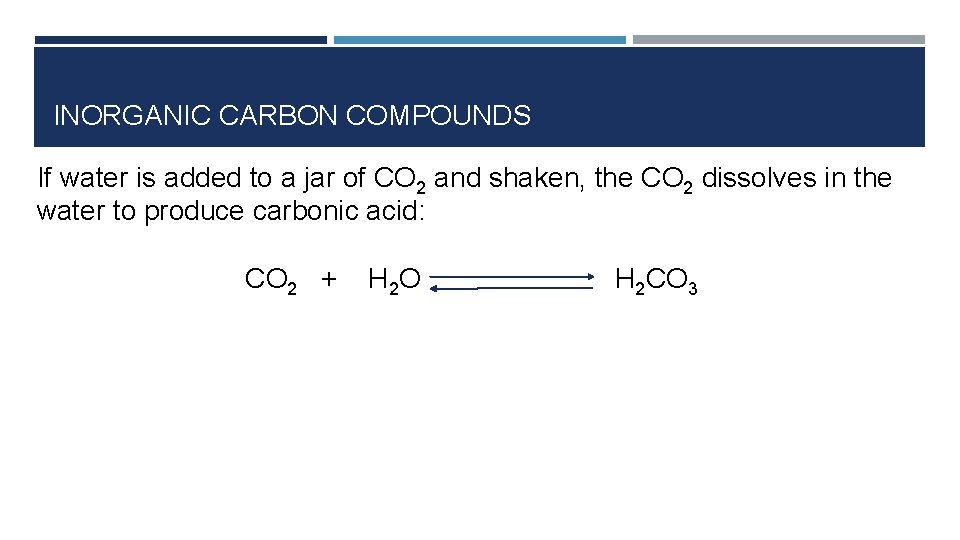
INORGANIC CARBON COMPOUNDS If water is added to a jar of CO 2 and shaken, the CO 2 dissolves in the water to produce carbonic acid: CO 2 + H 2 O H 2 CO 3
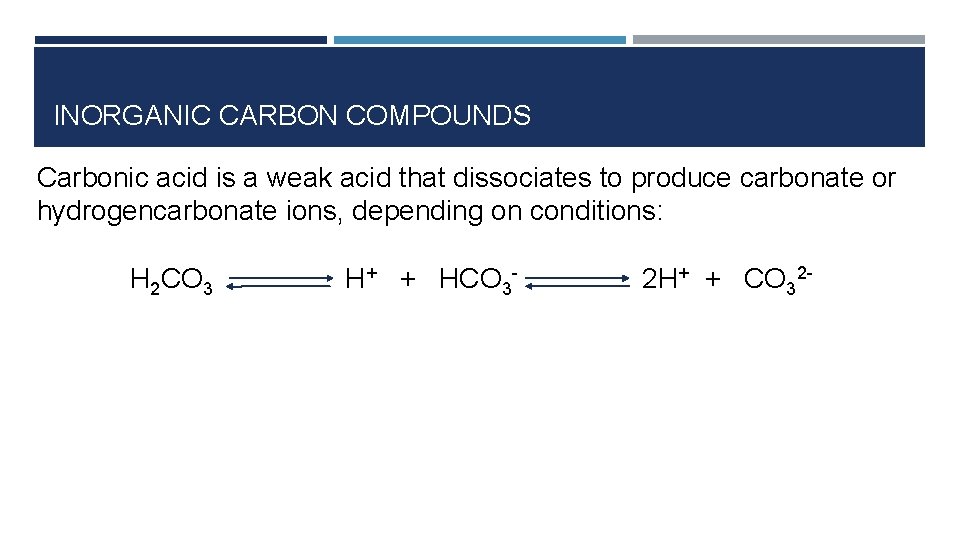
INORGANIC CARBON COMPOUNDS Carbonic acid is a weak acid that dissociates to produce carbonate or hydrogencarbonate ions, depending on conditions: H 2 CO 3 H+ + HCO 3 - 2 H+ + CO 32 -

INORGANIC CARBON COMPOUNDS • Pure carbonic acid cannot be isolated • Carbon dioxide may exist in its free state or as carbonate or hydrogencarbonate salts • Calcium carbonate (Ca. CO 3) is one of the most common substances found in nature (marble, chalk and limestone)

INORGANIC CARBON COMPOUNDS • Carbon dioxide is said to be an acidic oxide, because it forms an acidic solution with water

INORGANIC CARBON COMPOUNDS • Common use of carbon dioxide = putting fizz in drinks • First fizzy drink made in 1766 by Joseph Priestly (the discoverer of oxygen)
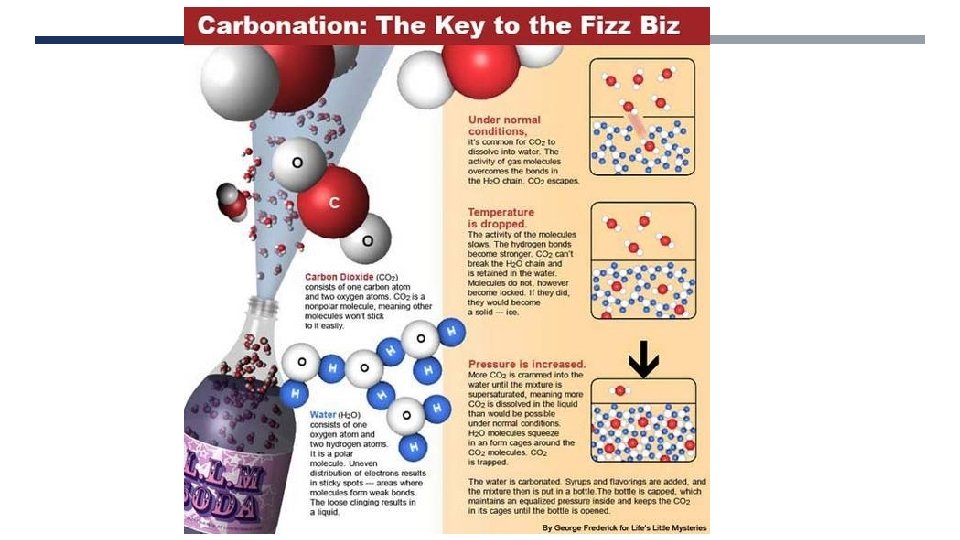

INORGANIC CARBON COMPOUNDS • Carbon dioxide used in fire extinguishers • Carbon dioxide can be pressurised and cooled to -78 °C to make ‘dry ice’ which is used in stage effects and to transport items which need to be kept cold
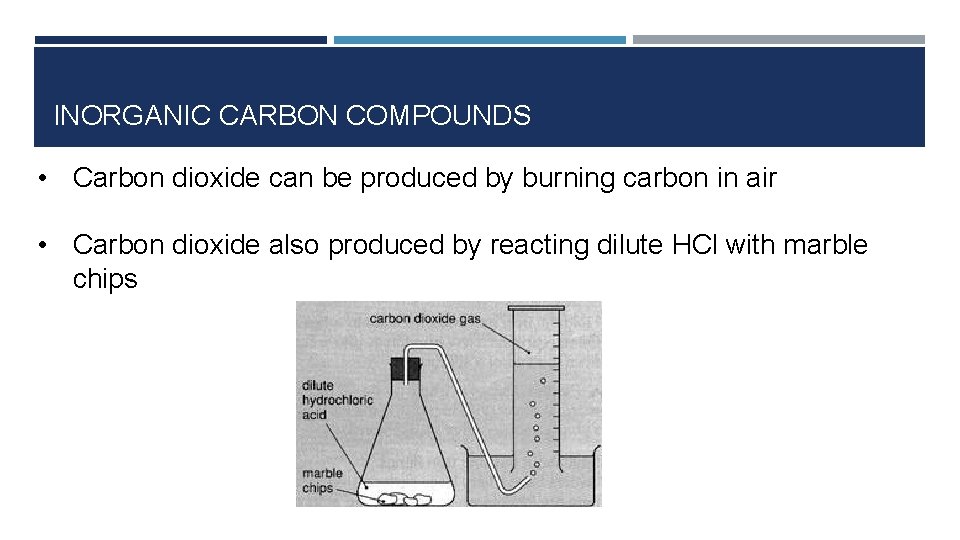
INORGANIC CARBON COMPOUNDS • Carbon dioxide can be produced by burning carbon in air • Carbon dioxide also produced by reacting dilute HCl with marble chips
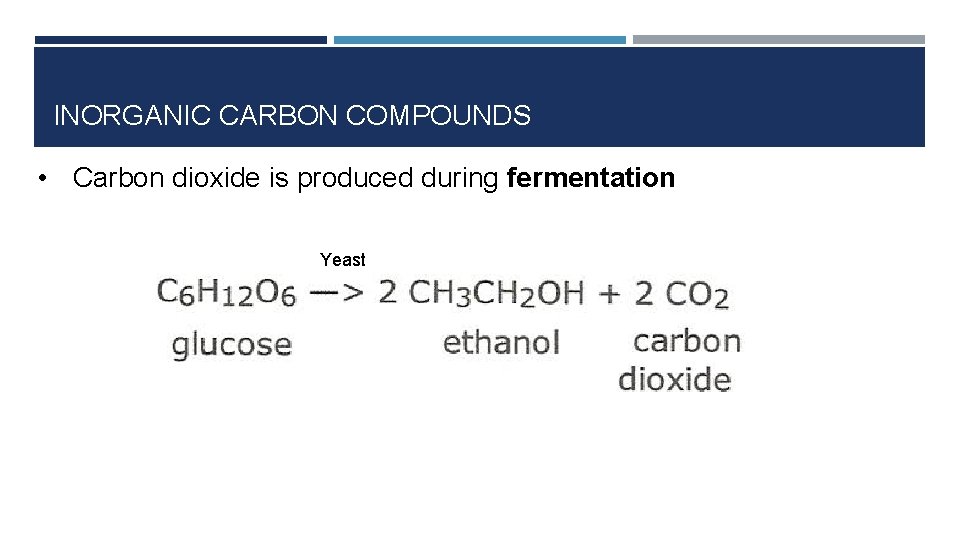
INORGANIC CARBON COMPOUNDS • Carbon dioxide is produced during fermentation Yeast
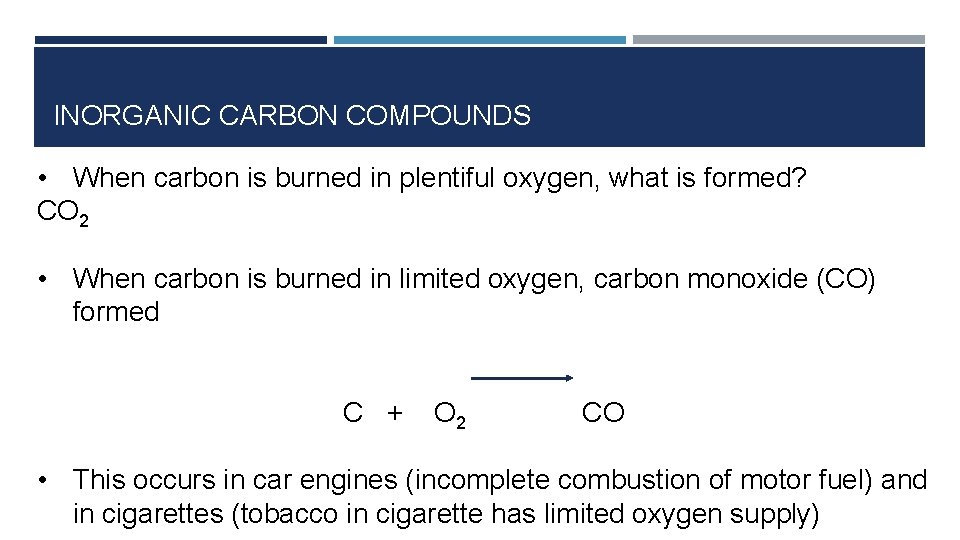
INORGANIC CARBON COMPOUNDS • When carbon is burned in plentiful oxygen, what is formed? CO 2 • When carbon is burned in limited oxygen, carbon monoxide (CO) formed C + O 2 CO • This occurs in car engines (incomplete combustion of motor fuel) and in cigarettes (tobacco in cigarette has limited oxygen supply)

INORGANIC CARBON COMPOUNDS Carbon monoxide • Colourless gas with no smell. It is a neutral oxide • Highly poisonous as it binds to haemoglobin and reduces its ability to carry oxygen • Catalytic converters in cars convert carbon monoxide to carbon dioxide • Must always ensure there is a good air supply when fuel is being

INORGANIC CARBON COMPOUNDS
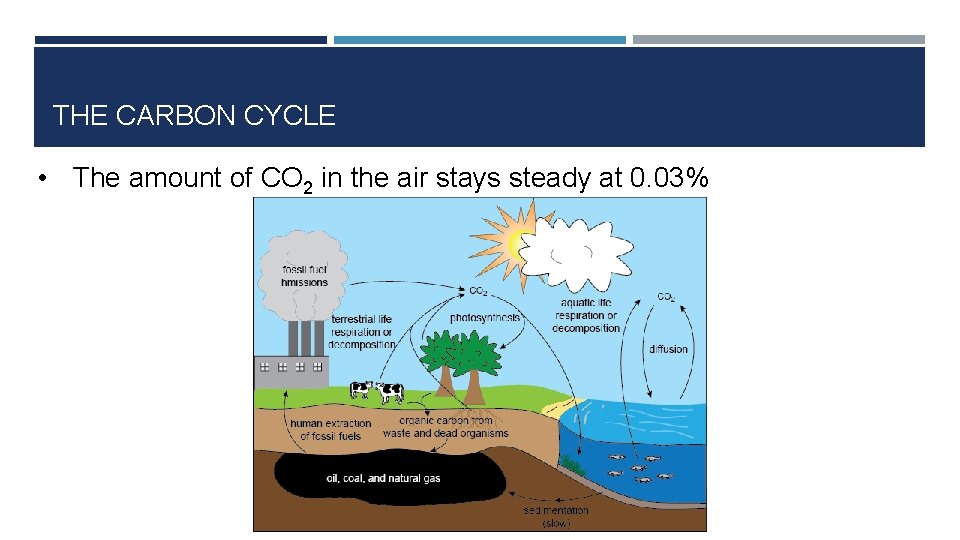
THE CARBON CYCLE • The amount of CO 2 in the air stays steady at 0. 03%

THE CARBON CYCLE • Carbon dioxide removed from the air by photosynthesis • Carbon dioxide also removed by dissolving in oceans, rainwater etc. • Carbon dioxide added to the air by respiration (we each breathe out about 500 L of carbon dioxide per day!) • Carbon dioxide also added to air by burning fossil fuels (global
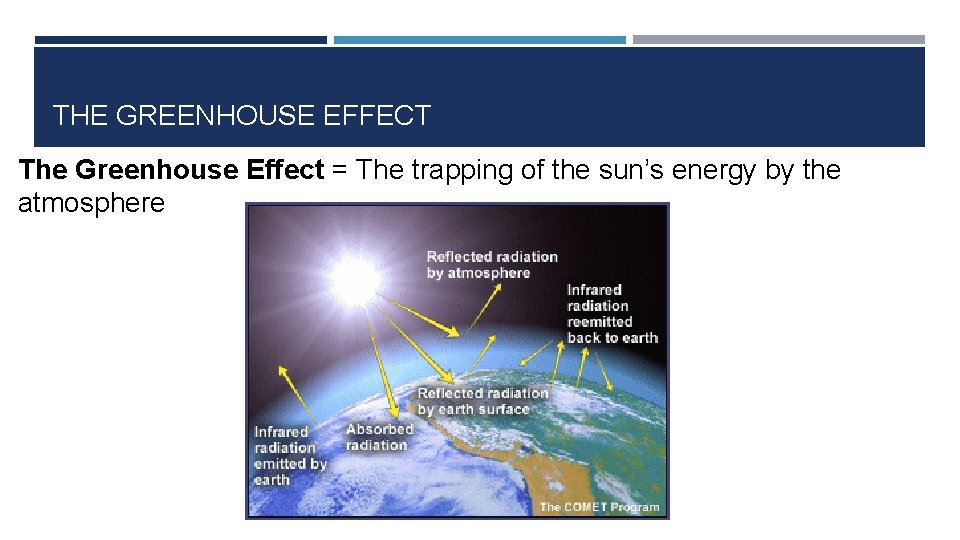
THE GREENHOUSE EFFECT The Greenhouse Effect = The trapping of the sun’s energy by the atmosphere
THE GREENHOUSE EFFECT • The greenhouse effect is a natural occurrence • Without the greenhouse effect, the average air temperature on Earth would drop from 15 °C to -15 °C! • The moon is the same distance from the sun as Earth but it has no atmosphere. The average temperature there is -18 °C.

THE GREENHOUSE EFFECT • Greenhouse gases = gases that are good at absorbing heat given off from the Earth Can you name some greenhouse gases? • Main greenhouse gases = carbon dioxide and water • Other greenhouse gases = methane, nitrous oxide and CFCs
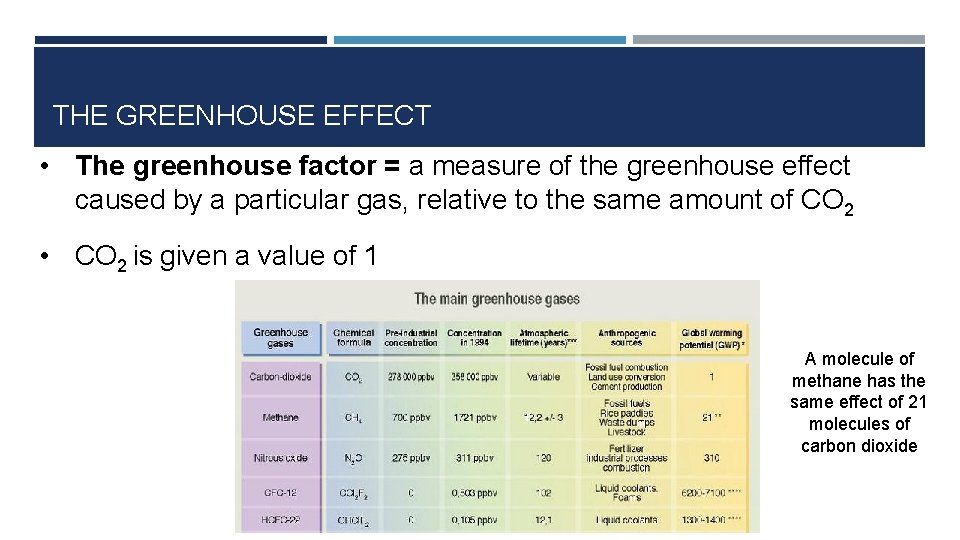
THE GREENHOUSE EFFECT • The greenhouse factor = a measure of the greenhouse effect caused by a particular gas, relative to the same amount of CO 2 • CO 2 is given a value of 1 A molecule of methane has the same effect of 21 molecules of carbon dioxide
THE GREENHOUSE EFFECT • Increasing concentrations of greenhouse gases in the atmosphere are causing an enhanced greenhouse effect • This causes extra warming which we call global warming
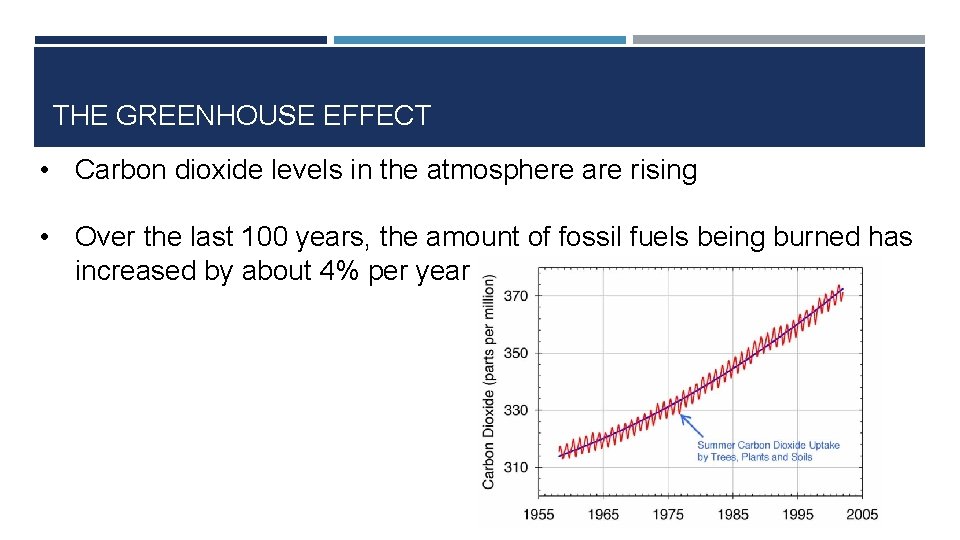
THE GREENHOUSE EFFECT • Carbon dioxide levels in the atmosphere are rising • Over the last 100 years, the amount of fossil fuels being burned has increased by about 4% per year
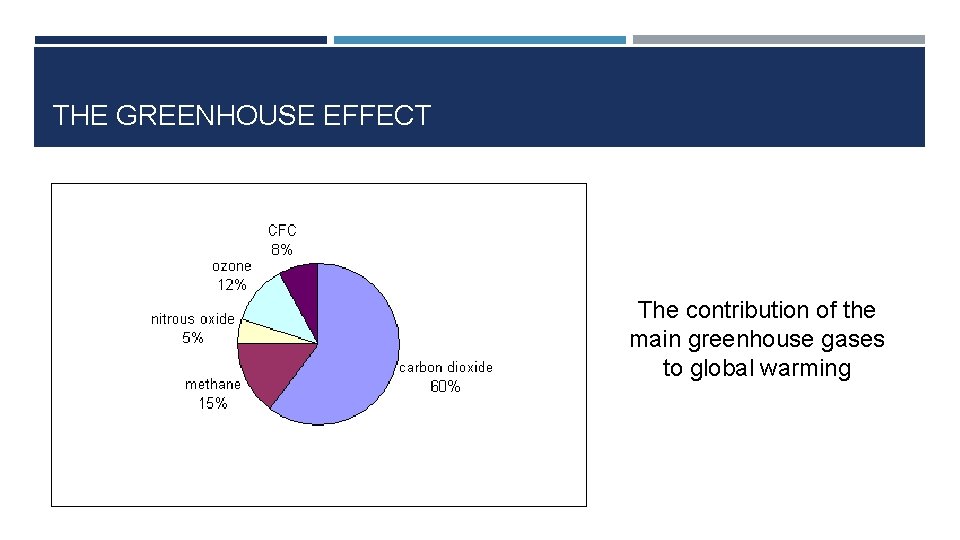
THE GREENHOUSE EFFECT The contribution of the main greenhouse gases to global warming

THE GREENHOUSE EFFECT • Water vapour is also a greenhouse gas and has a greater overall effect than carbon dioxide because of its higher concentration • You may have noticed that nights are cold when there are no clouds! • Human activity has little effect on water vapour levels in the atmosphere

THE GREENHOUSE EFFECT • The turnover of CO 2 in the atmosphere is very slow • The rate of turnover = the residence time • Some CO 2 dissolves in seawater but can take 100 s of years to reach the bottom of the ocean and precipitate as calcium carbonate. • CO 2 has a residence time of ~100 years in the atmosphere • Photosynthesis cannot remove CO 2 quickly enough from the atmosphere

THE GREENHOUSE EFFECT Other greenhouse gases affected by human activity: Methane: Landfill rubbish dumps, ruminants, natural gas leakage etc. CFCs: Fridges, foams, aerosol sprays etc Nitrous oxide: Car exhaust fumes, nitrogenous fertilisers etc • Residence time of methane = ~10 years • Residence time of CFCs and nitrous oxide = ~100 years

THE GREENHOUSE EFFECT How do we prevent global warming? • Laws to limit CO 2 emissions and CFC use • Protect tropical rainforests

THE GREENHOUSE EFFECT What are the implications of the enhanced greenhouse effect? 1. Rise in sea level - Glaciers are melting and water in the ocean is expanding 2. Climate changes - More violent weather disturbances 3. Agriculture - Crops will grow more quickly but increased rainfall could decrease crop yield
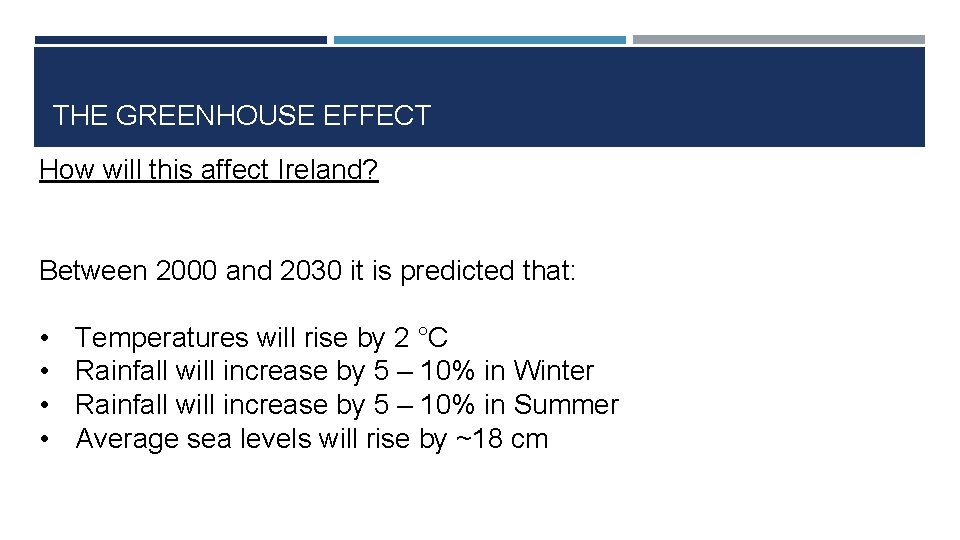
THE GREENHOUSE EFFECT How will this affect Ireland? Between 2000 and 2030 it is predicted that: • • Temperatures will rise by 2 °C Rainfall will increase by 5 – 10% in Winter Rainfall will increase by 5 – 10% in Summer Average sea levels will rise by ~18 cm

THE GREENHOUSE EFFECT Question Distinguish between the terms “greenhouse effect” and “enhanced greenhouse effect. ”

ATMOSPHERIC POLLUTION Air pollution = a situation that exists when a constituent in the air is present to the extent that there is a significant hazard to present or future health or to the environment

ATMOSPHERIC POLLUTION • Not all gases released by human activity damage the environment We will examine two types of pollutants: 1. Gases which give rise to acid rain 2. Gases that damage the ozone layer

ATMOSPHERIC POLLUTION 1. Gases which cause acid rain • Sulfur dioxide and nitrogen dioxide are the main contributors to acid rain • Normal rainwater is slightly acidic. Why? • Acid rain can have a p. H of about 2 - 5

ACID RAIN Sulfur dioxide (SO 2) • Some sulphur dioxide comes from natural sources (volcanoes, rotting plants etc) • 85% of sulphur dioxide comes from burning fossil fuels • Sulphur in the fuel combines with O 2 in the air to produce SO 2
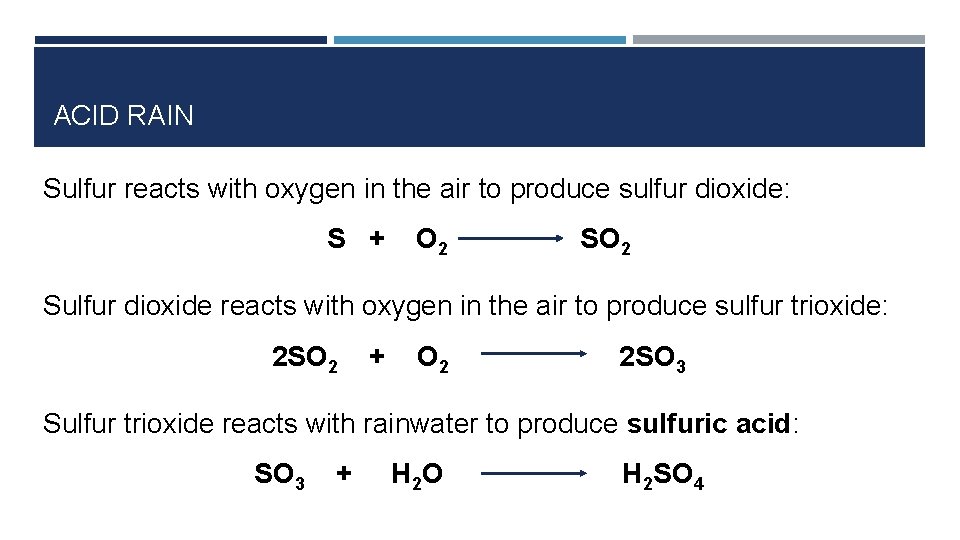
ACID RAIN Sulfur reacts with oxygen in the air to produce sulfur dioxide: S + O 2 Sulfur dioxide reacts with oxygen in the air to produce sulfur trioxide: 2 SO 2 + O 2 2 SO 3 Sulfur trioxide reacts with rainwater to produce sulfuric acid: SO 3 + H 2 O H 2 SO 4

ACID RAIN Nitrogen compounds • Oxides of nitrogen also cause acid rain • Main oxides of nitrogen in the atmosphere are: nitrogen monoxide (NO) and nitrogen dioxide (NO 2) • They are produced naturally by soil bacteria and lightning discharges
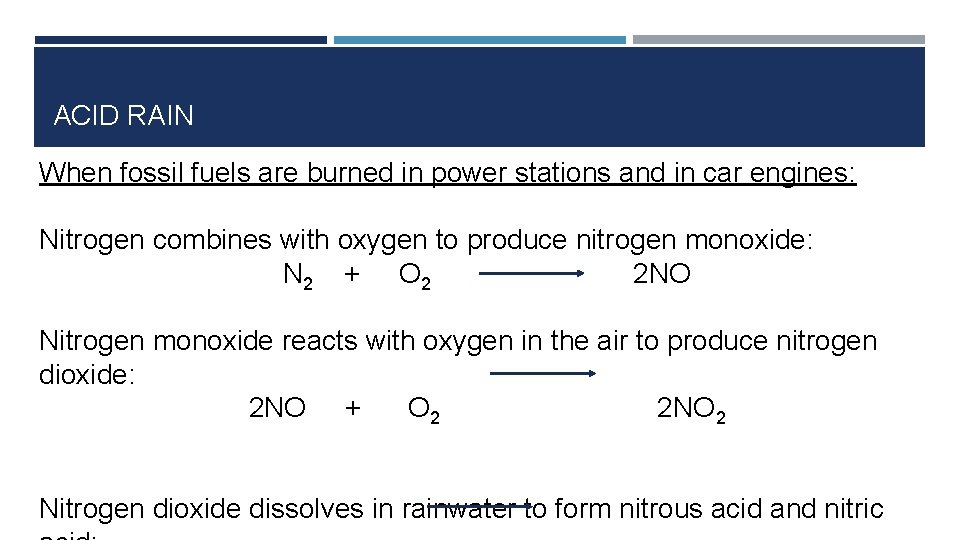
ACID RAIN When fossil fuels are burned in power stations and in car engines: Nitrogen combines with oxygen to produce nitrogen monoxide: N 2 + O 2 2 NO Nitrogen monoxide reacts with oxygen in the air to produce nitrogen dioxide: 2 NO + O 2 2 NO 2 Nitrogen dioxide dissolves in rainwater to form nitrous acid and nitric

ACID RAIN Effects of acid rain: • Damages trees • Kills fish in lakes • Washes essential nutrients out of soil • Damages limestone buildings

ACID RAIN How to combat acid rain • Power stations have “scrubbing systems” to remove sulphur dioxide from chimneys • Limestone is often used as a scrubber: Ca. CO 3 + SO 2 Limestone + sulfur dioxide Ca. SO 3 + CO 2 calcium sulfite + carbon dioxide • Calcium sulfite reacts with oxygen to form calcium sulfate used to make

THE OZONE LAYER Ozone = O 3 • Ozone (trioxygen) is a pungent, pale blue gas which condenses to a deep blue liquid at -112 °C

THE OZONE LAYER • Ozone layer is 25 - 50 km above Earth • The concentration of ozone in the ozone layer is ~10 ppm • Ozone performs an important task in the stratosphere • Ozone absorbs harmful UV radiation from the sun
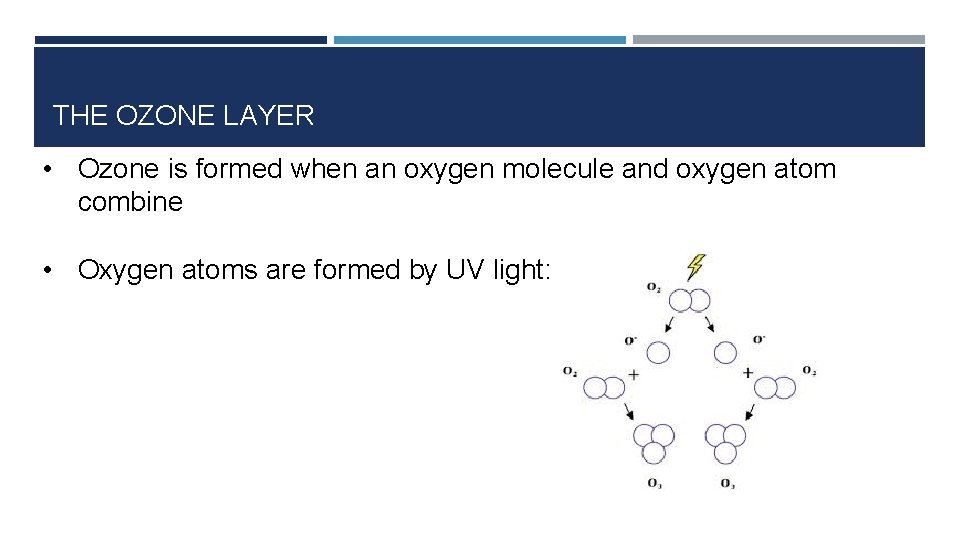
THE OZONE LAYER • Ozone is formed when an oxygen molecule and oxygen atom combine • Oxygen atoms are formed by UV light:

THE OZONE LAYER • The formation of oxygen atoms is an example of photodissociation (breaking a bond using radiation) • Oxygen atoms are very reactive because of unpaired electron • Oxygen atoms are often called oxygen free radicals • As soon as oxygen atoms are formed, they react with oxygen molecules to form ozone

THE OZONE LAYER • The energy required to break the oxygen molecules into oxygen atoms comes from UV radiation or electricity passing through air • You will often smell ozone near photocopiers or electric motors because high voltage produces ozone!

THE OZONE LAYER • Ozone absorbs UV radiation: • Most of the oxygen atoms produced re-form ozone • Some of the oxygen atoms produced destroy ozone molecules and convert them to oxygen:

THE OZONE LAYER • Ozone is being made and destroyed all the time • Ozone is not formed below the stratosphere because there isn’t enough high energy UV light available • The high energy UV light has already been absorbed by O 2 and O 3 in the stratosphere!

THE OZONE LAYER • In 1984 a hole was discovered in the ozone layer • There are holes in the ozone layer over the Antarctic, the Arctic and there is a general decrease in ozone levels around the globe • Chlorine atoms (produced by CFCs) are largely responsible for destroying the ozone layer
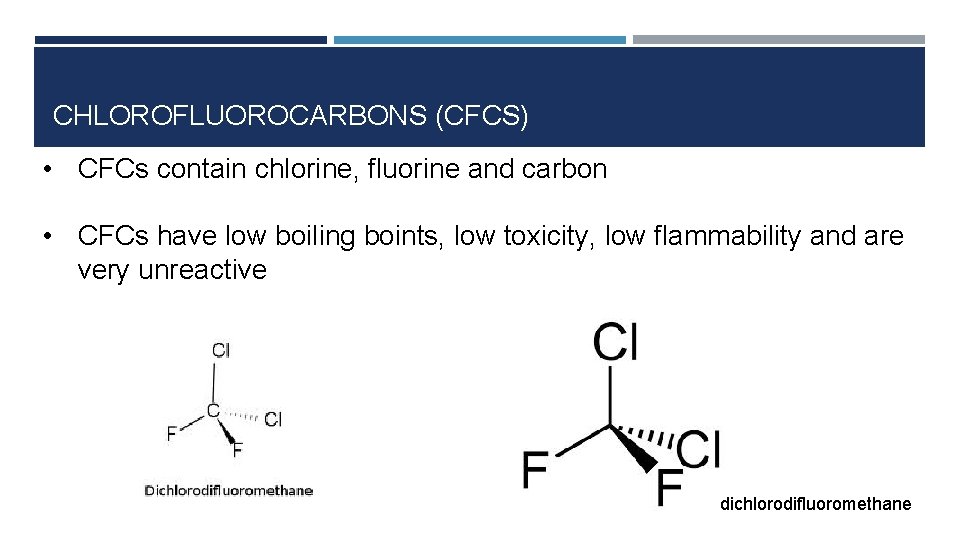
CHLOROFLUOROCARBONS (CFCS) • CFCs contain chlorine, fluorine and carbon • CFCs have low boiling boints, low toxicity, low flammability and are very unreactive dichlorodifluoromethane

CHLOROFLUOROCARBONS (CFCS) Main uses of CFCs: • Propellants in deodorants and polishes • Blowing agents in the manufacture of polystyrene • Fridges • Air conditioning units
CHLOROFLUOROCARBONS (CFCS) • CFCs have a residence time of ~100 years in the lower atmosphere • This gives plenty of time for them to move into the stratosphere where they become reactive • CFCs are broken down by UV radiation:

CHLOROFLUOROCARBONS (CFCS) • Chlorine atoms attack ozone to form oxygen and chlorine oxide: • Chlorine oxide attacks an oxygen atom: • A chain reaction occurs and one chlorine atom can destroy thousands of ozone molecules

CHLOROFLUOROCARBONS (CFCS) • An International Conference in Montreal in 1990 decide on a total phasing out of all CFCs by 2000 • CFCs are still present in large quantities in fridges, air conditioners etc. • Even if emission of CFCs was stopped immediately, damage to the ozone layer could continue for another 20 years

CHLOROFLUOROCARBONS (CFCS) • Why does the hole in the ozone layer appear over the Antarctic? • The hole appears during Spring in the Antarctic (Sept – Nov) • During Winter months, clouds of icy particles appear in the stratosphere • Harmless chlorine molecule attach to the icy particles • Reactions occur to produce Cl 2 molecules

CHLOROFLUOROCARBONS (CFCS) Nitrogen monoxide • Nitrogen monoxide also destroys ozone: • Nitrogen monoxide comes from reactions in bacteria an lightening • CFCs cause more damage to ozone layer than nitrogen monoxide

CHLOROFLUOROCARBONS (CFCS) Preventing Depletion of the Ozone Layer • Methane reacts with chlorine atoms to prevent them destroying ozone: • The HCl produced is removed in raindrops to get rid of some chlorine in the atmosphere
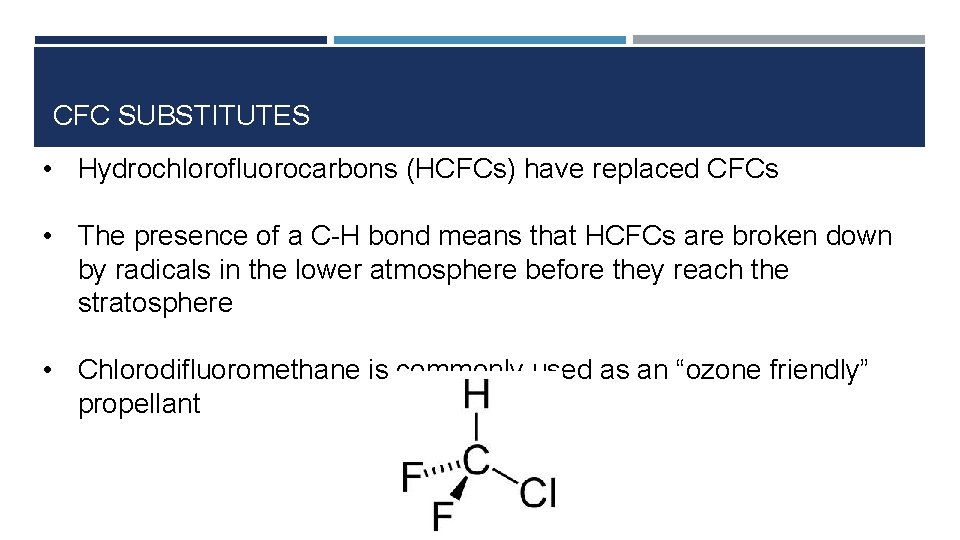
CFC SUBSTITUTES • Hydrochlorofluorocarbons (HCFCs) have replaced CFCs • The presence of a C-H bond means that HCFCs are broken down by radicals in the lower atmosphere before they reach the stratosphere • Chlorodifluoromethane is commonly used as an “ozone friendly” propellant
CFC SUBSTITUTES • HCFCs are not the perfect solution • They destroy ozone but to the same extent as CFCs • Some HCFCs can have a damaging effect of up to 33% of that of CFCs • HCFCs are greenhouse gases • Current research is focussing on developing hydrofluorocarbons which have no chlorines in their molecules
- Slides: 74