Atmosphere Composition pressure and heat Use these slides


- Slides: 14

Atmosphere: Composition, pressure and heat Use these slides as a guide to fill in your atmosphere packet! Answer the questions at the end and do the last page

The Atmosphere A layer of gas that surrounds Earth. Created long ago by volcanic eruptions. Held around Earth by gravity. Regulates Earth’s temperature. Mr. Fetch’s Earth Science Classroom

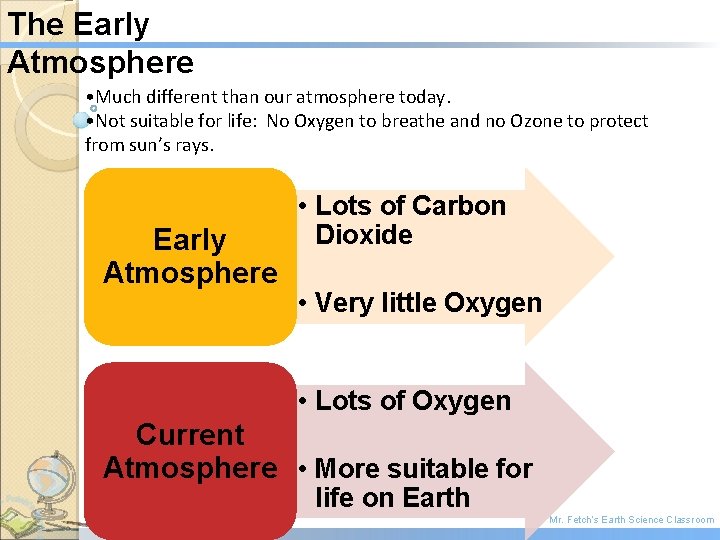
The Early Atmosphere • Much different than our atmosphere today. • Not suitable for life: No Oxygen to breathe and no Ozone to protect from sun’s rays. Early Atmosphere • Lots of Carbon Dioxide • Very little Oxygen • Lots of Oxygen Current Atmosphere • More suitable for life on Earth Mr. Fetch’s Earth Science Classroom

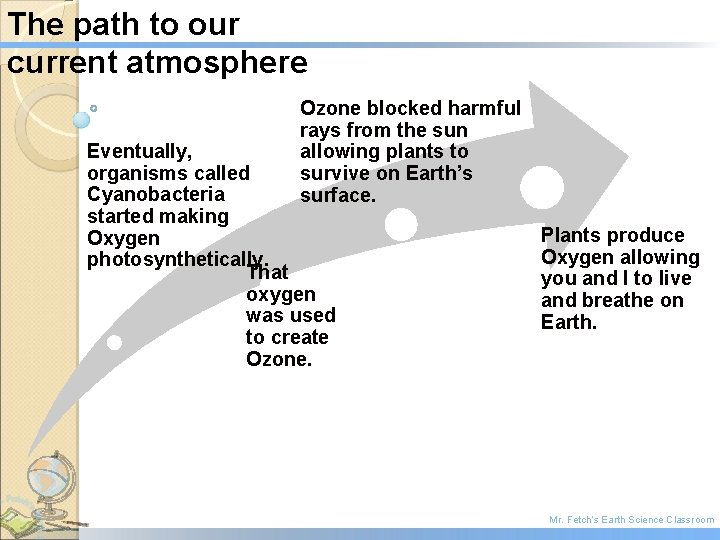
The path to our current atmosphere Ozone blocked harmful rays from the sun allowing plants to survive on Earth’s surface. Eventually, organisms called Cyanobacteria started making Oxygen photosynthetically. That oxygen was used to create Ozone. Plants produce Oxygen allowing you and I to live and breathe on Earth. Mr. Fetch’s Earth Science Classroom

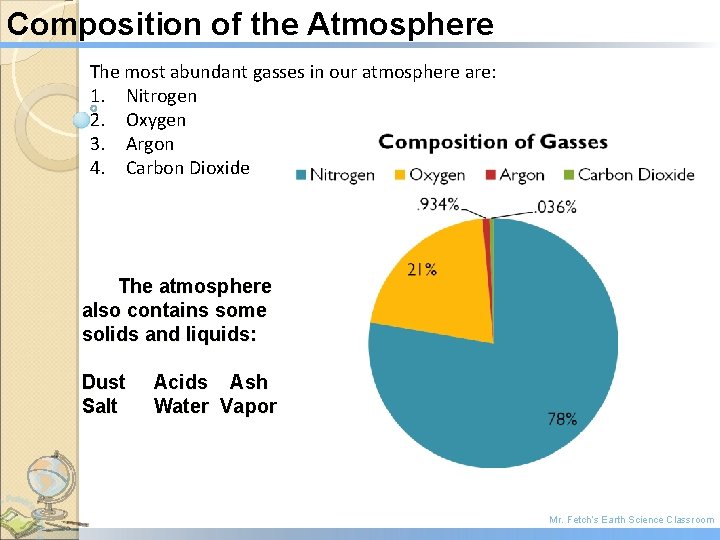
Composition of the Atmosphere The most abundant gasses in our atmosphere are: 1. Nitrogen 2. Oxygen 3. Argon 4. Carbon Dioxide The atmosphere also contains some solids and liquids: Dust Salt Acids Ash Water Vapor Mr. Fetch’s Earth Science Classroom

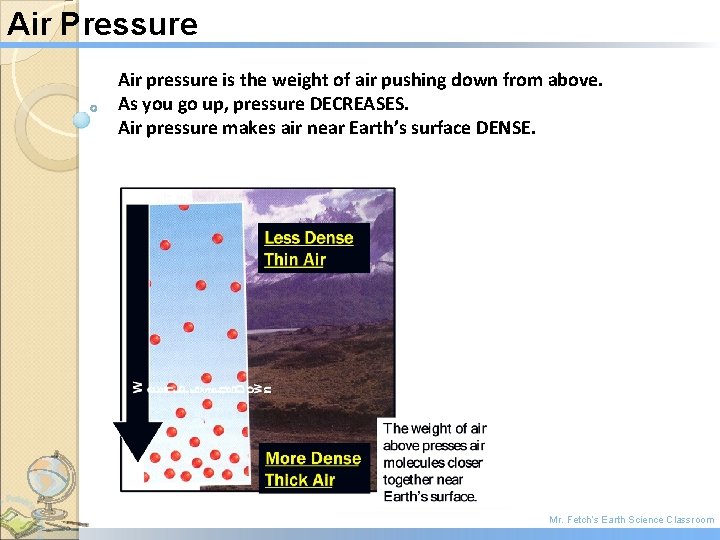
Air Pressure Air pressure is the weight of air pushing down from above. As you go up, pressure DECREASES. Air pressure makes air near Earth’s surface DENSE. Mr. Fetch’s Earth Science Classroom

Energy in the Atmosphere Mr. Fetch’s Earth Science Classroom

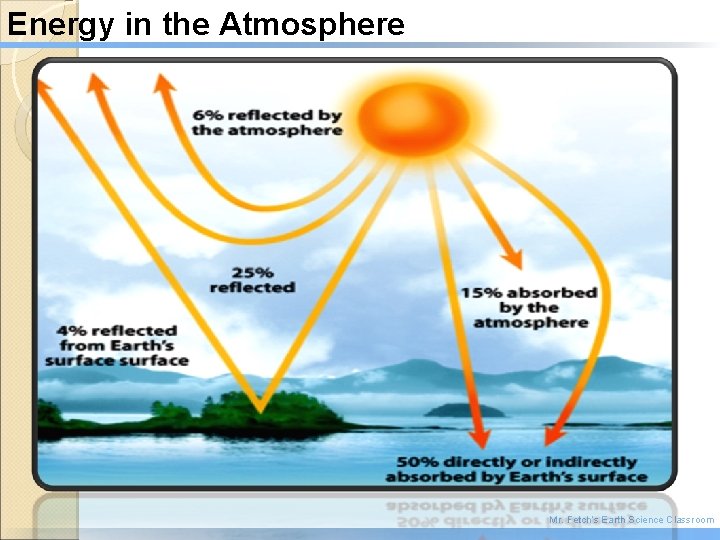
Energy in the Atmosphere �About 30% of the incoming sunlight is “scattered or reflected, ” reflecting light back into space, �Scattering is what makes the sky blue, not black! �About 70% of incoming sunlight is absorbed by the atmosphere or Earth’s surface, this is used and converted into heat.

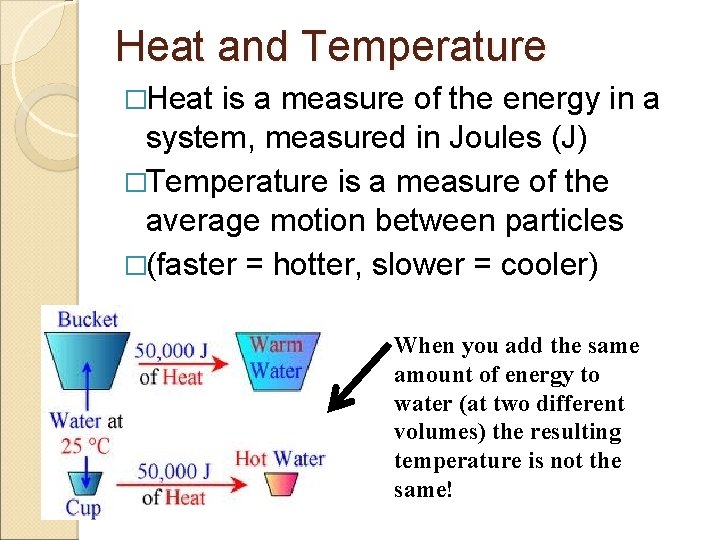
Heat and Temperature �Heat is a measure of the energy in a system, measured in Joules (J) �Temperature is a measure of the average motion between particles �(faster = hotter, slower = cooler) When you add the same amount of energy to water (at two different volumes) the resulting temperature is not the same!

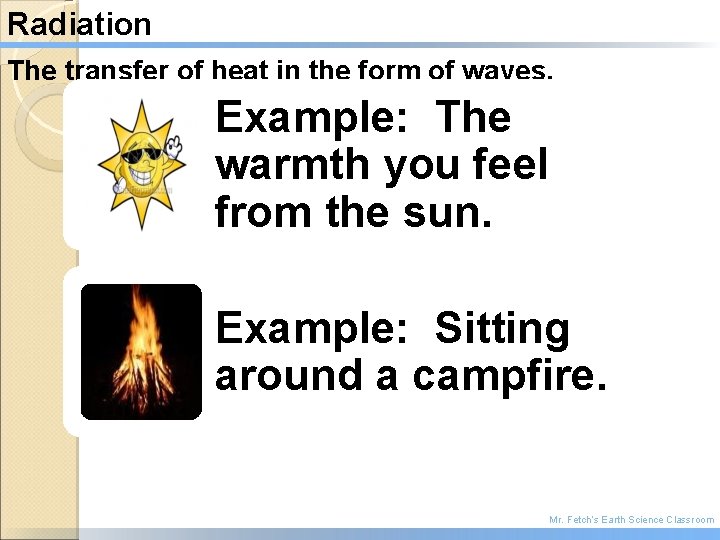
Radiation The transfer of heat in the form of waves. Example: The warmth you feel from the sun. Example: Sitting around a campfire. Mr. Fetch’s Earth Science Classroom

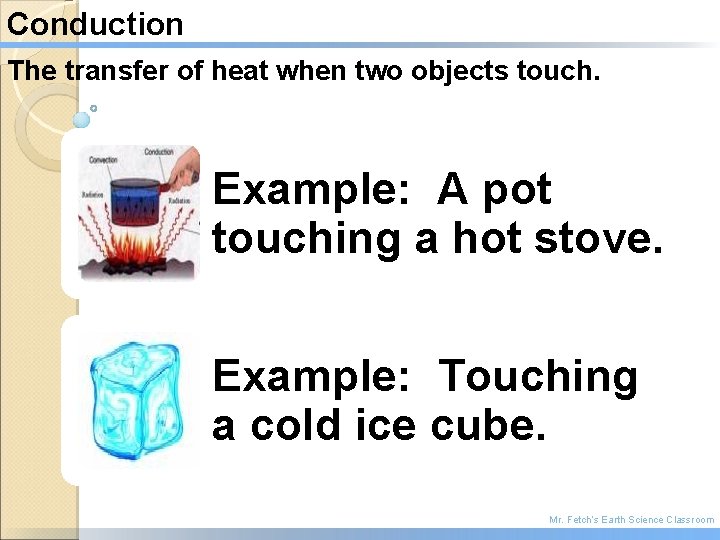
Conduction The transfer of heat when two objects touch. Example: A pot touching a hot stove. Example: Touching a cold ice cube. Mr. Fetch’s Earth Science Classroom

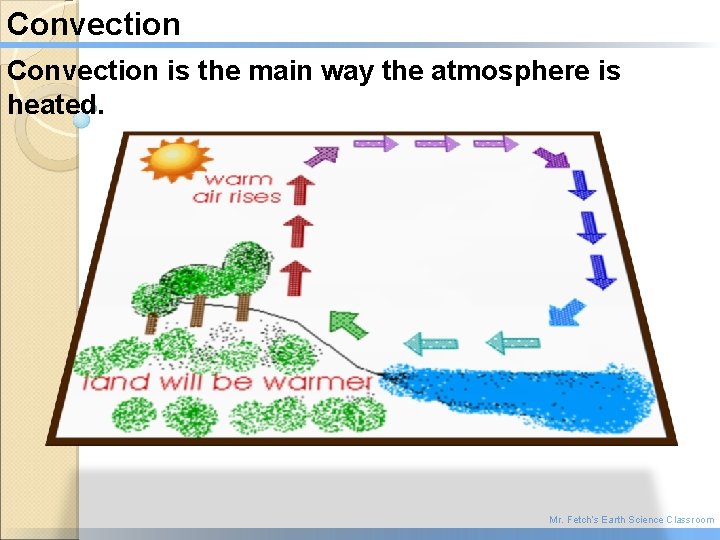
Convection The transfer of heat through liquids or gasses. - Warm air rising - Cold air sinking Example: Heating up water on a stove Example: Heating a room in your house Mr. Fetch’s Earth Science Classroom

Convection is the main way the atmosphere is heated. Mr. Fetch’s Earth Science Classroom

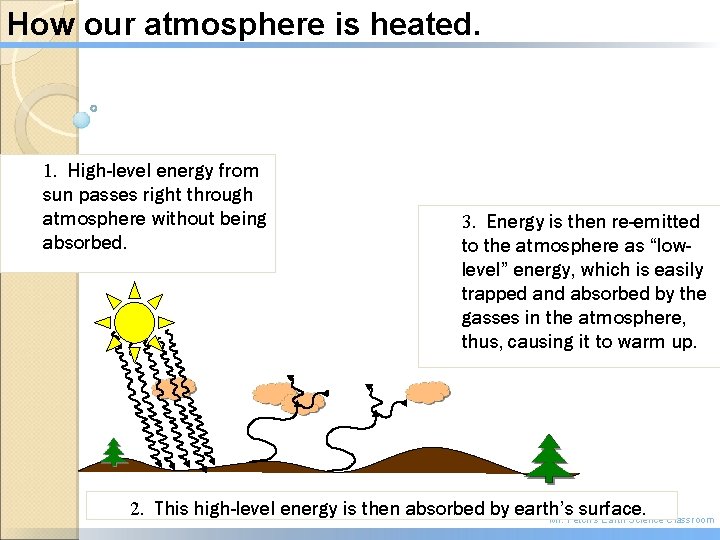
How our atmosphere is heated. 1. High-level energy from sun passes right through atmosphere without being absorbed. 3. Energy is then re-emitted to the atmosphere as “lowlevel” energy, which is easily trapped and absorbed by the gasses in the atmosphere, thus, causing it to warm up. 2. This high-level energy is then absorbed by earth’s surface. Mr. Fetch’s Earth Science Classroom