At Home With Growing Older An Interdisciplinary Inquiry



![PET: PIB-PET analysis • Post [11 C]PIB injection (~15 m. Ci) • ECAT EXACT PET: PIB-PET analysis • Post [11 C]PIB injection (~15 m. Ci) • ECAT EXACT](https://slidetodoc.com/presentation_image_h/fc0db81caf78a95ca9ec904582dd8c34/image-27.jpg)





- Slides: 50

At Home With Growing Older: An Interdisciplinary Inquiry On Aging January 21, 2016 Sleeping well to live well: The role of sleep in normal and abnormal aging Bryce Mander, Ph. D Sleep and Neuroimaging Laboratory Department of Psychology & Helen Wills Neuroscience Institute University of California, Berkeley

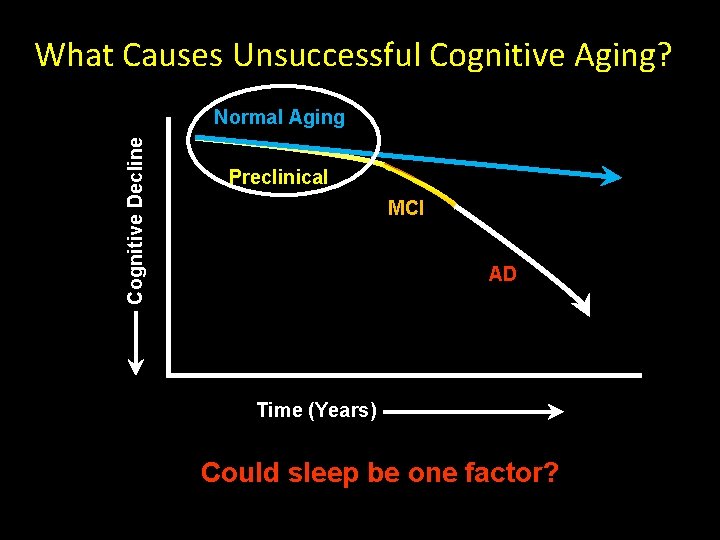
What Causes Unsuccessful Cognitive Aging? Cognitive Decline Normal Aging Preclinical MCI AD Time (Years) Could sleep be one factor?

What is sleep? Sleep is a behavior found in many species – Behavioral quiescence – Recumbent posture – Reduced responsiveness to the environment – Deprivation is followed by “rebound” Associated with complex changes in brain functioning, e. g. changes in EEG recordings

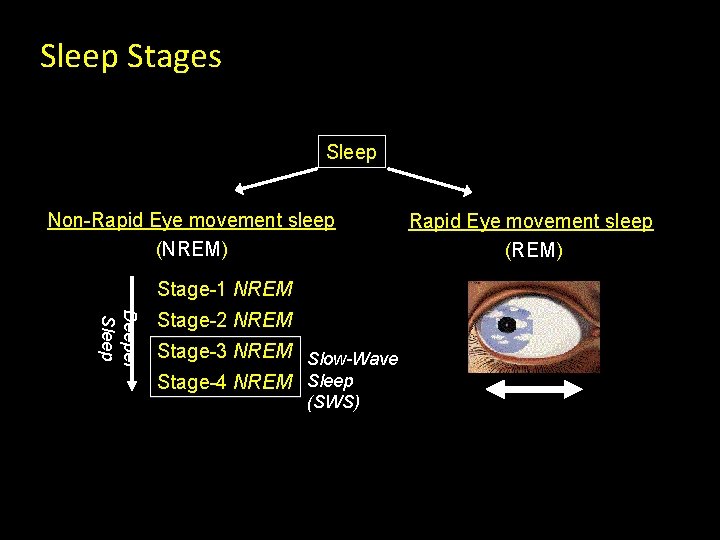
Sleep Stages Sleep Non-Rapid Eye movement sleep (NREM) Stage-1 NREM Deeper Sleep Stage-2 NREM Stage-3 NREM Slow-Wave Stage-4 NREM Sleep (SWS) Rapid Eye movement sleep (REM)

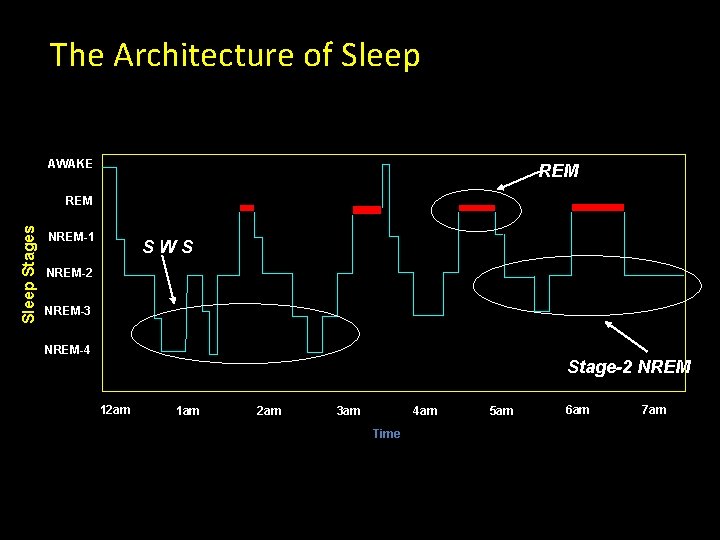
The Architecture of Sleep AWAKE REM Sleep Stages REM NREM-1 SWS NREM-2 NREM-3 NREM-4 Stage-2 NREM 12 am 1 am 2 am 4 am 3 am Time 5 am 6 am 7 am

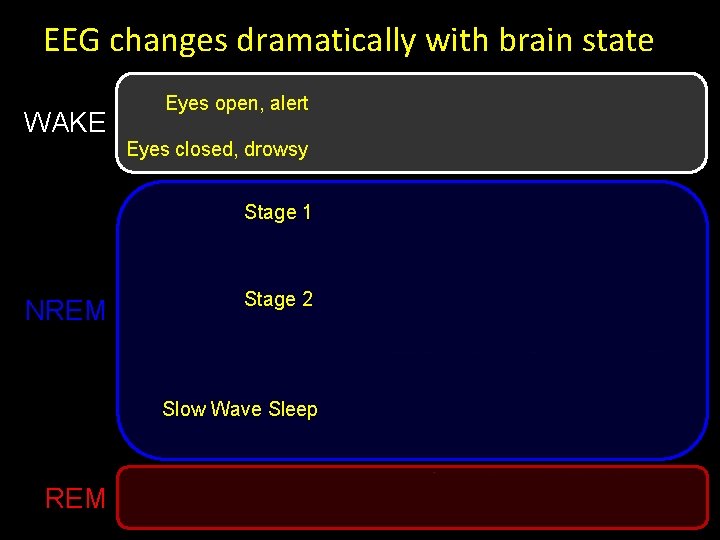
EEG changes dramatically with brain state WAKE Eyes open, alert Eyes closed, drowsy Stage 1 NREM Stage 2 Slow Wave Sleep REM

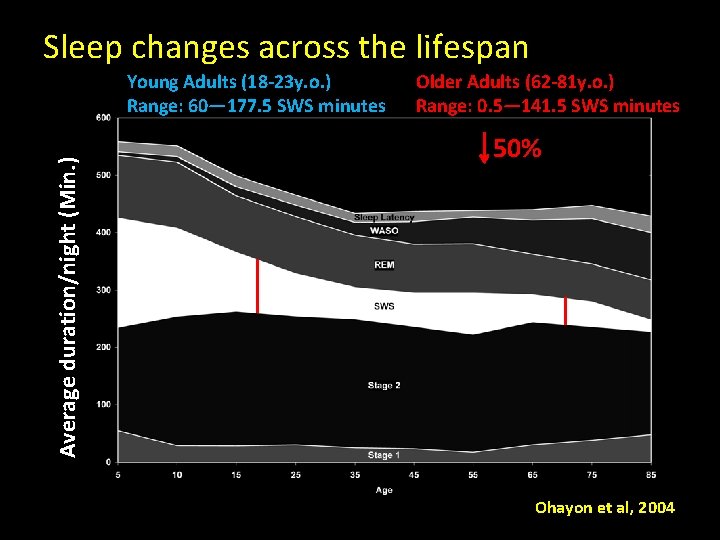
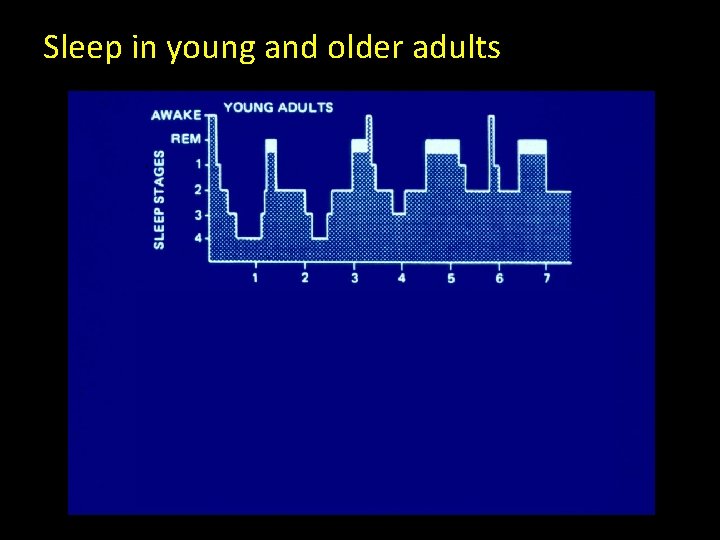
Sleep changes across the lifespan Average duration/night (Min. ) Young Adults (18 -23 y. o. ) Range: 60— 177. 5 SWS minutes Older Adults (62 -81 y. o. ) Range: 0. 5— 141. 5 SWS minutes 50% Ohayon et al, 2004

Slow waves change with age Slow Wave Properties 1 2 3 NREM Period 4 1 2 3 4 NREM Period From Carrier et al, 2011

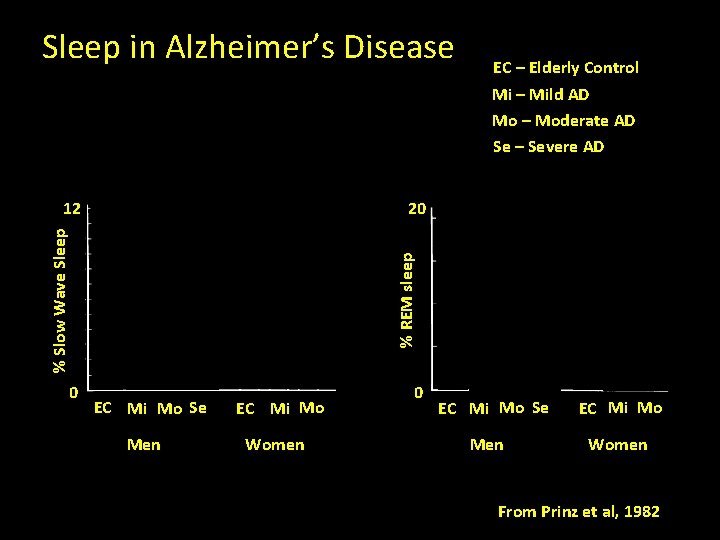
Sleep in Alzheimer’s Disease EC – Elderly Control Mi – Mild AD Mo – Moderate AD Se – Severe AD 20 0 % REM sleep % Slow Wave Sleep 12 EC Mi Mo Se Men EC Mi Mo Women 0 EC Mi Mo Se Men EC Mi Mo Women From Prinz et al, 1982

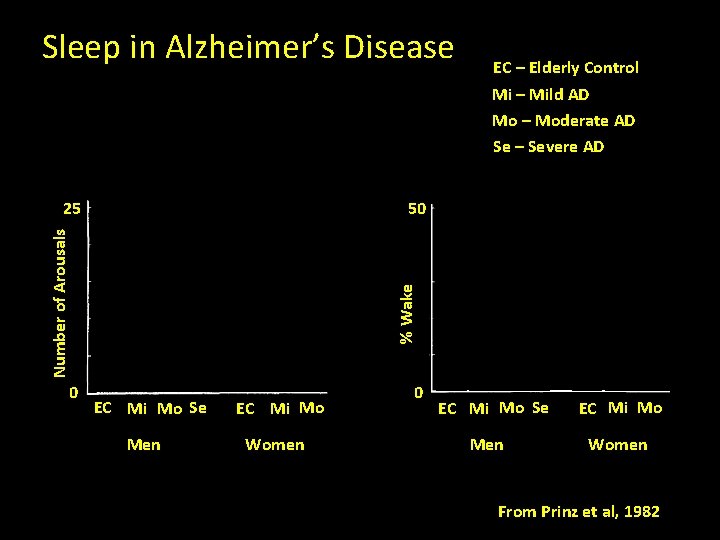
Sleep in Alzheimer’s Disease EC – Elderly Control Mi – Mild AD Mo – Moderate AD Se – Severe AD 50 0 % Wake Number of Arousals 25 EC Mi Mo Se Men EC Mi Mo Women 0 EC Mi Mo Se Men EC Mi Mo Women From Prinz et al, 1982

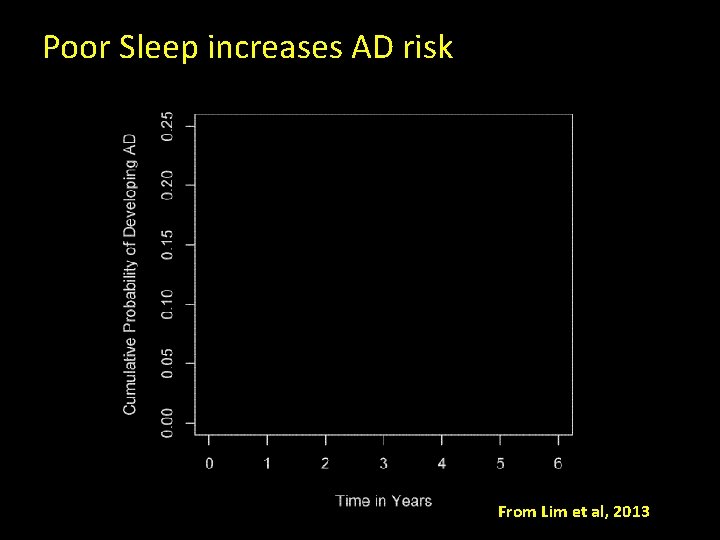
Poor Sleep increases AD risk High sleep fragmentation Low sleep fragmentation From Lim et al, 2013

Sleep disorders and AD • Sleep disorders are more common in old age – but there is wide variability (Vitiello 2009) • In dementia – >60% have at least one (Guarnieri 2012, Ancoli-Israel 1991) – sleep apnea and insomnia are most common • Sleep apnea and insomnia – increases AD risk (Yaffe 2011, Osorio 2011) – lowers age of AD onset (Osorio 2015) – treatment delays age of AD onset (Osorio 2015)

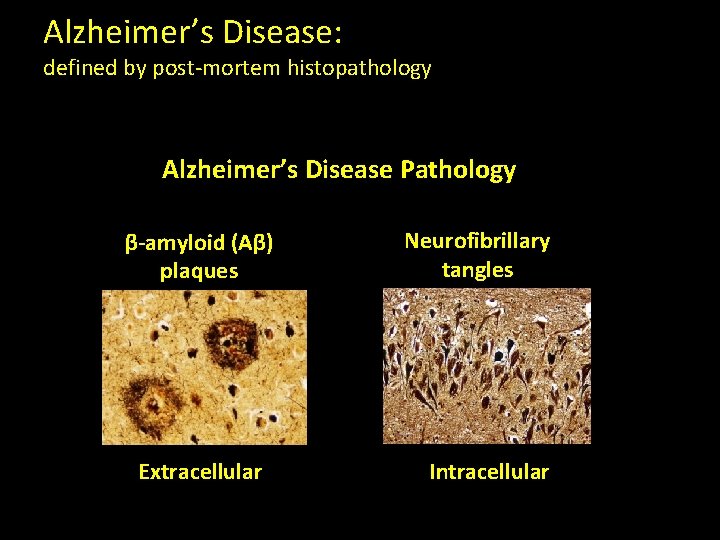
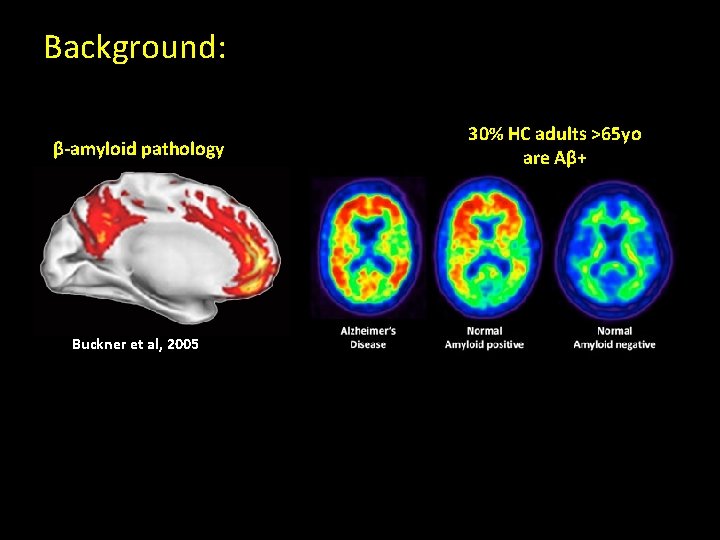
Alzheimer’s Disease: defined by post-mortem histopathology Alzheimer’s Disease Pathology β-amyloid (Aβ) plaques Extracellular Neurofibrillary tangles Intracellular

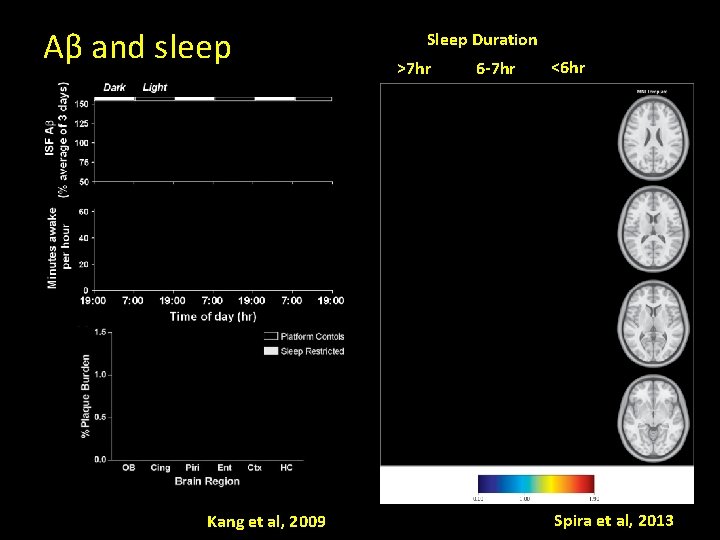
Aβ and sleep Kang et al, 2009 Sleep Duration >7 hr 6 -7 hr <6 hr Spira et al, 2013

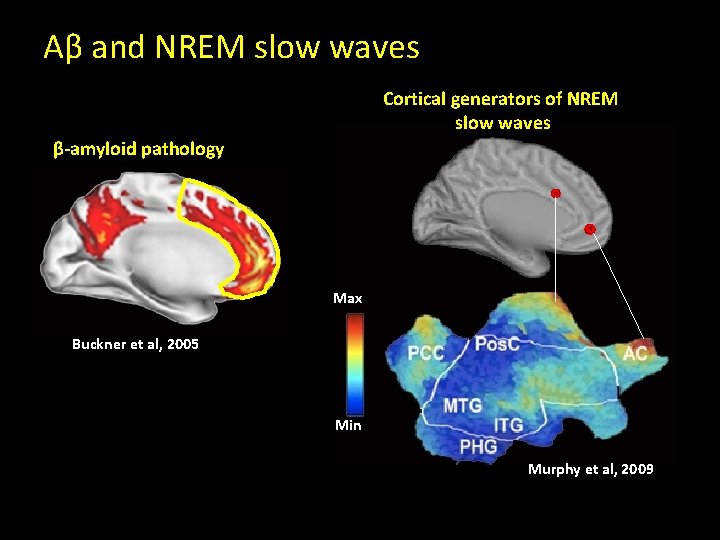
Aβ and NREM slow waves Cortical generators of NREM slow waves β-amyloid pathology Max Buckner et al, 2005 Min Murphy et al, 2009

Specific aim 1: β-amyloid pathology Disrupted SWA Functional Consequence?

Stages of Memory Formation Encoding Consolidation Aging impairs memory Craik, 1977 Grady et al, 1995 Schacter et al, 1996 Albert, 1997 Daselaar et al, 2003 Davis et al, 2003 Recall Time Sperling et al, 2003 Buckner, 2004 Hedden et al, 2004 Miller et al, 2008 Mormino et al, 2009 Van Der Werf et al, 2010

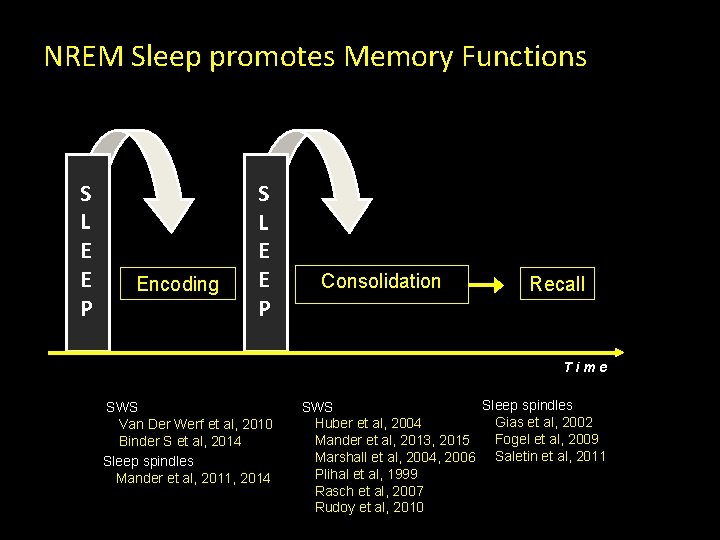
NREM Sleep promotes Memory Functions S L E E P Encoding S L E E P Consolidation Recall Time SWS Van Der Werf et al, 2010 Binder S et al, 2014 Sleep spindles Mander et al, 2011, 2014 Sleep spindles SWS Gias et al, 2002 Huber et al, 2004 Fogel et al, 2009 Mander et al, 2013, 2015 Marshall et al, 2004, 2006 Saletin et al, 2011 Plihal et al, 1999 Rasch et al, 2007 Rudoy et al, 2010

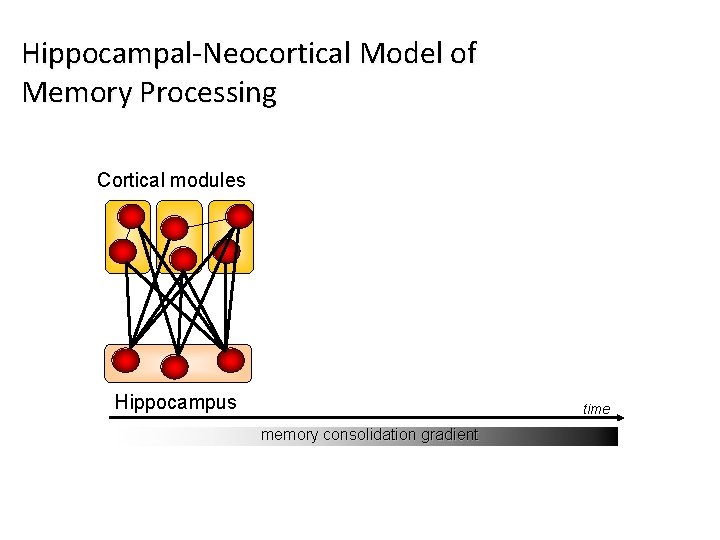
Hippocampal-Neocortical Model of Memory Processing Cortical modules Hippocampus time memory consolidation gradient

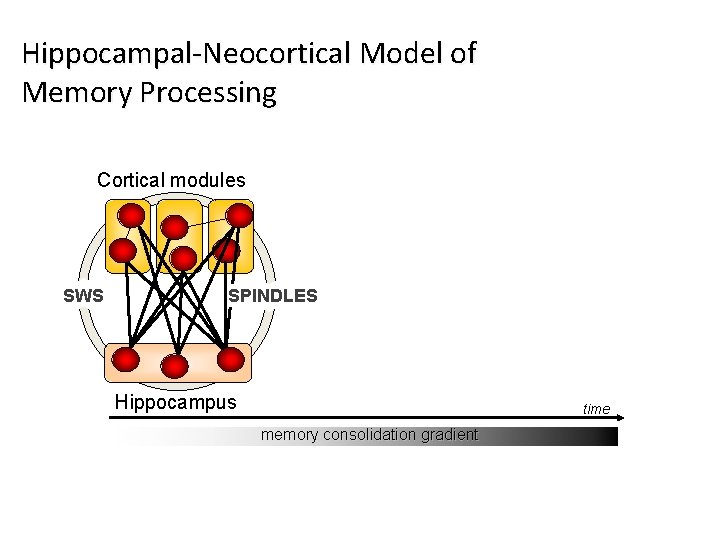
Hippocampal-Neocortical Model of Memory Processing Cortical modules SWS SPINDLES Hippocampus time memory consolidation gradient

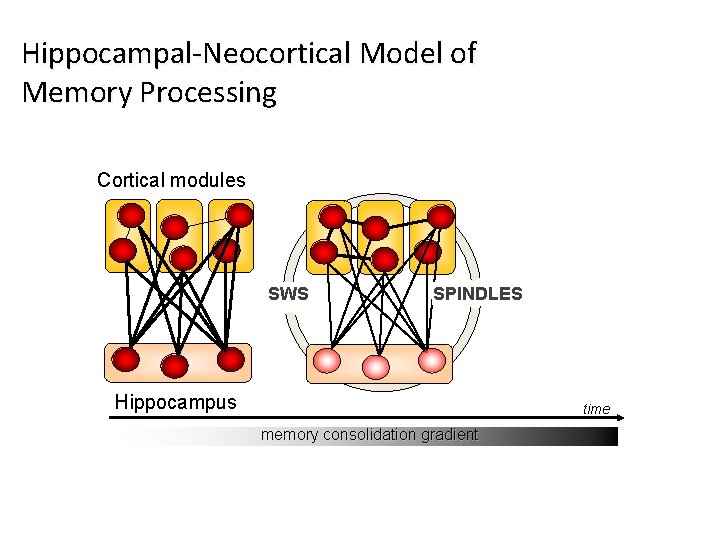
Hippocampal-Neocortical Model of Memory Processing Cortical modules SWS SPINDLES Hippocampus time memory consolidation gradient

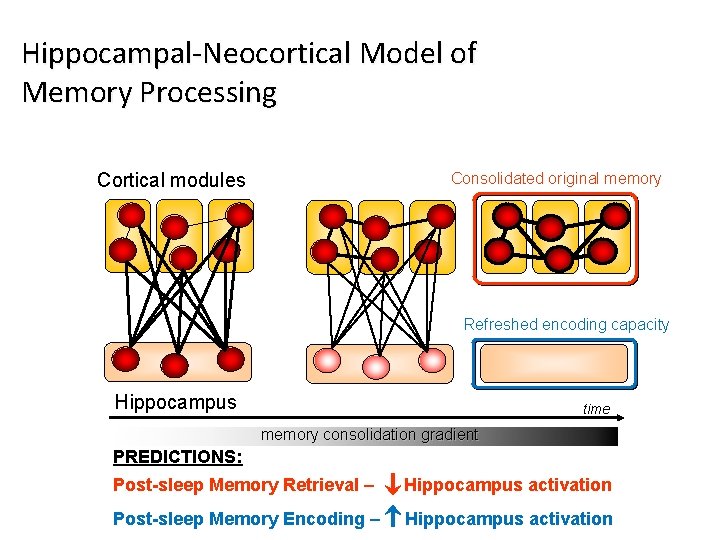
Hippocampal-Neocortical Model of Memory Processing Cortical modules Consolidated original memory Refreshed encoding capacity Hippocampus time memory consolidation gradient PREDICTIONS: Post-sleep Memory Retrieval – Hippocampus activation Post-sleep Memory Encoding – Hippocampus activation

Specific aim 2: Memory

Participants: • Twenty-six healthy older adults – 75. 1± 3. 5 years, 18 Female • No neurological, cognitive, psychiatric or sleep disorders • performed within age-matched norms on battery of neuropsychologal tests • Kept regular sleep schedule for 5 days prestudy • Drug, alcohol and caffeine free during experiment

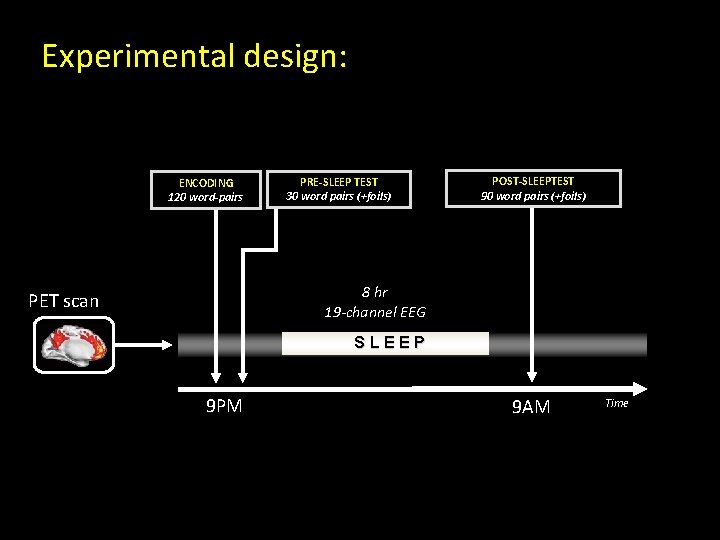
Experimental design: ENCODING 120 word-pairs PRE-SLEEP TEST 30 word pairs (+foils) POST-SLEEPTEST 90 word pairs (+foils) 8 hr 19 -channel EEG PET scan SLEEP 9 PM 9 AM Time

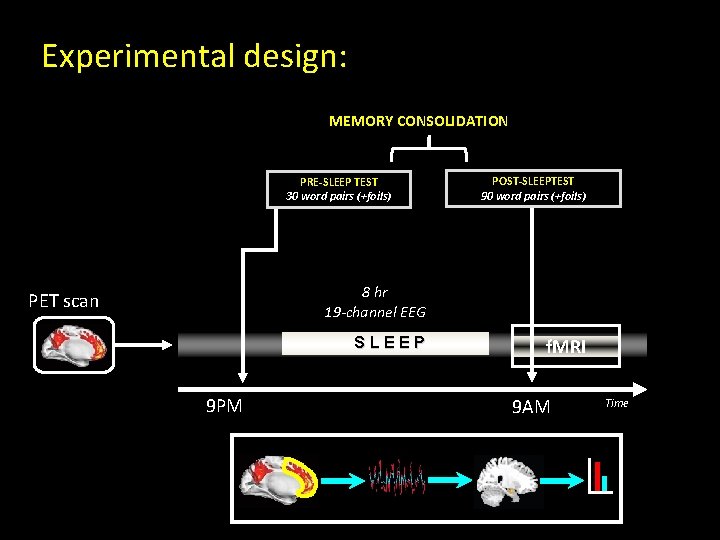
Experimental design: MEMORY CONSOLIDATION PRE-SLEEP TEST 30 word pairs (+foils) POST-SLEEPTEST 90 word pairs (+foils) 8 hr 19 -channel EEG PET scan SLEEP 9 PM f. MRI 9 AM Time
![PET PIBPET analysis Post 11 CPIB injection 15 m Ci ECAT EXACT PET: PIB-PET analysis • Post [11 C]PIB injection (~15 m. Ci) • ECAT EXACT](https://slidetodoc.com/presentation_image_h/fc0db81caf78a95ca9ec904582dd8c34/image-27.jpg)
PET: PIB-PET analysis • Post [11 C]PIB injection (~15 m. Ci) • ECAT EXACT HR PET scanner • Standard preprocessing in SPM 8 • Derived voxel-wise distribution volume ratio (DVR) maps – Cerebellar reference region – Corrected for partial volume effects • • m. PFC ROI derived from Desikan. Killian PIB DVR values LN transformed to minimize skewness

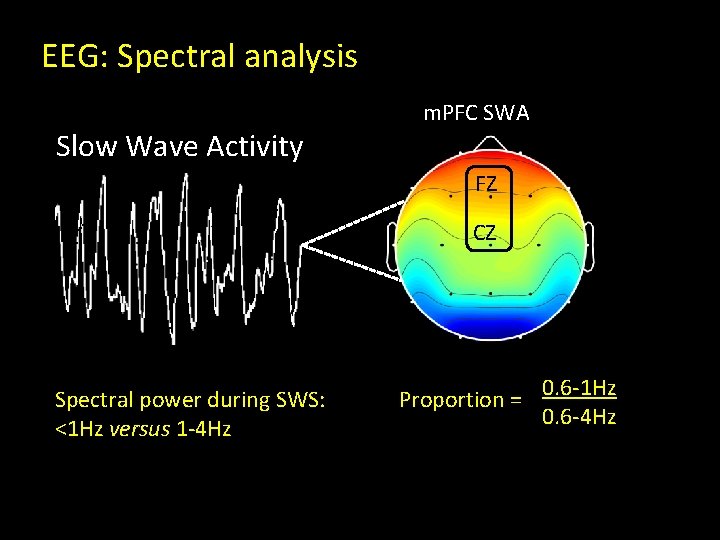
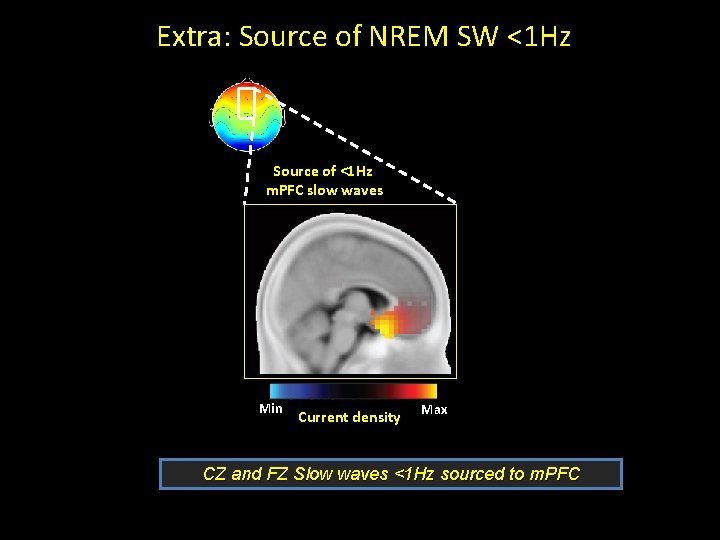
EEG: Spectral analysis Slow Wave Activity m. PFC SWA FZ CZ Spectral power during SWS: <1 Hz versus 1 -4 Hz Proportion = 0. 6 -1 Hz 0. 6 -4 Hz

f. MRI analysis • Siemens Trio 3 T MRI scanner • Standard preprocessing in SPM 8 • Normalized using template containing elderly brains • 8 mm Hippocampal ROI (Kim et al, 2011; P<0. 05 FWE corrected) • Retrieval-related activation [Hits-Correct Rejections] • Regression: Proportion of SWA <1 Hz
Results:

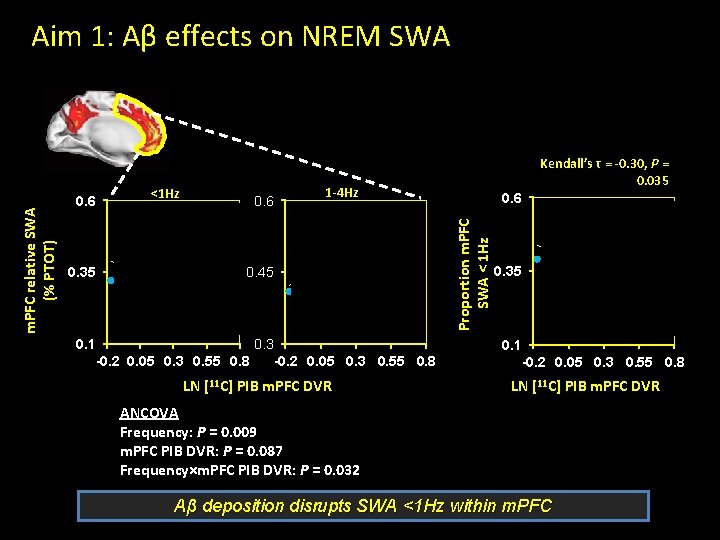
0. 6 <1 Hz 0. 6 r=-0. 42 P=0. 031 0. 35 0. 45 0. 1 0. 3 -0. 2 0. 05 0. 3 0. 55 0. 8 Kendall’s τ = -0. 30, P = 0. 035 1 -4 Hz 0. 6 r=0. 35 P=0. 076 Proportion m. PFC SWA < 1 Hz m. PFC relative SWA (% PTOT) Aim 1: Aβ effects on NREM SWA 0. 35 0. 1 -0. 2 0. 05 0. 3 0. 55 0. 8 LN [11 C] PIB m. PFC DVR ANCOVA Frequency: P = 0. 009 m. PFC PIB DVR: P = 0. 087 Frequency×m. PFC PIB DVR: P = 0. 032 Aβ deposition disrupts SWA <1 Hz within m. PFC

Aim 1: 2: Memory

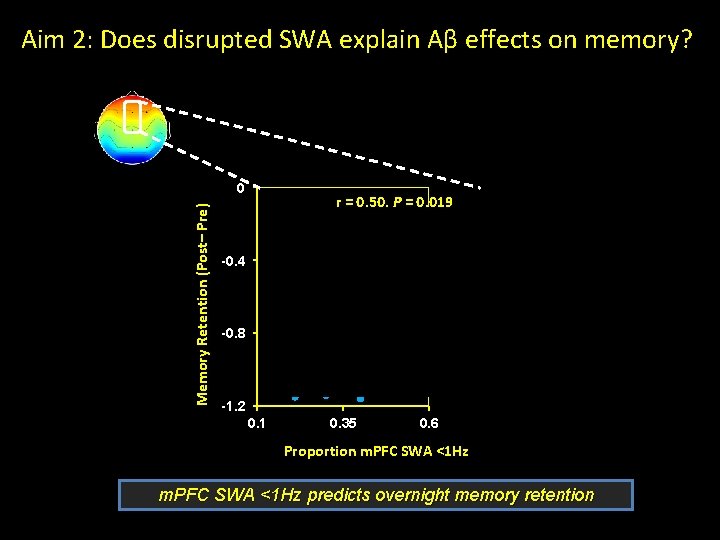
Aim 2: Does disrupted SWA explain Aβ effects on memory? Memory Retention (Post– Pre) 0 r = 0. 50, P = 0. 019 -0. 4 -0. 8 -1. 2 0. 1 0. 35 0. 6 Proportion m. PFC SWA <1 Hz predicts overnight memory retention

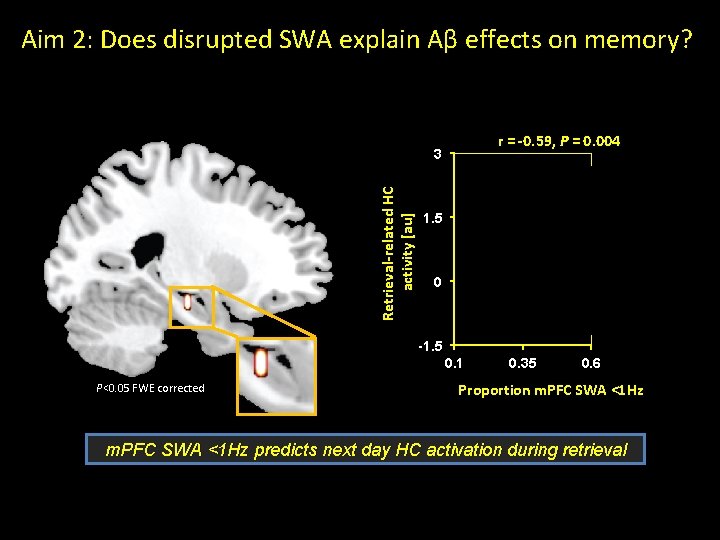
Aim 2: Does disrupted SWA explain Aβ effects on memory? r = -0. 59, P = 0. 004 Retrieval-related HC activity [au] 3 1. 5 0 -1. 5 0. 1 P<0. 05 FWE corrected 0. 35 0. 6 Proportion m. PFC SWA <1 Hz predicts next day HC activation during retrieval

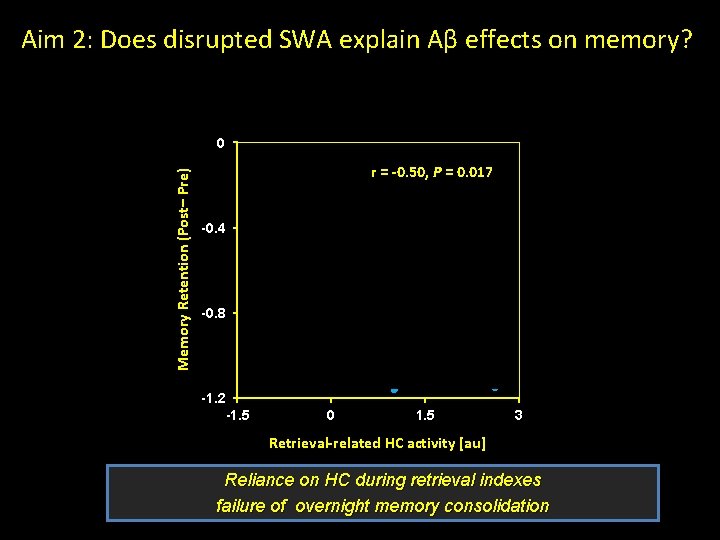
Aim 2: Does disrupted SWA explain Aβ effects on memory? Memory Retention (Post– Pre) 0 r = -0. 50, P = 0. 017 -0. 4 -0. 8 -1. 2 -1. 5 0 1. 5 3 Retrieval-related HC activity [au] Reliance on HC during retrieval indexes failure of overnight memory consolidation

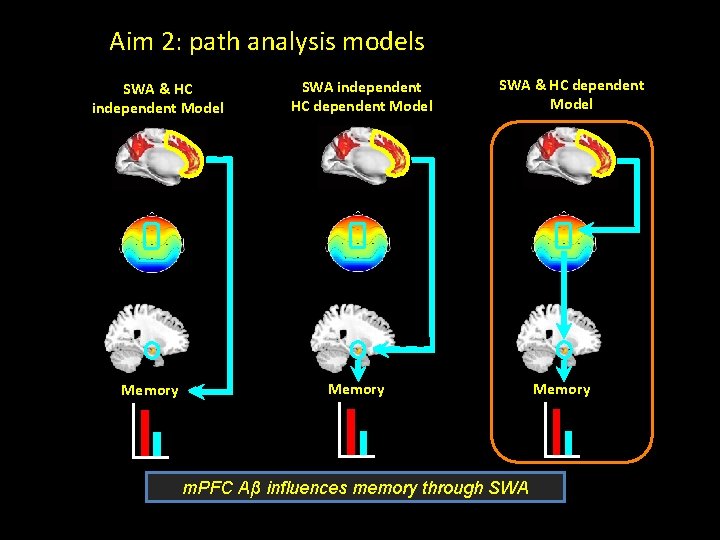
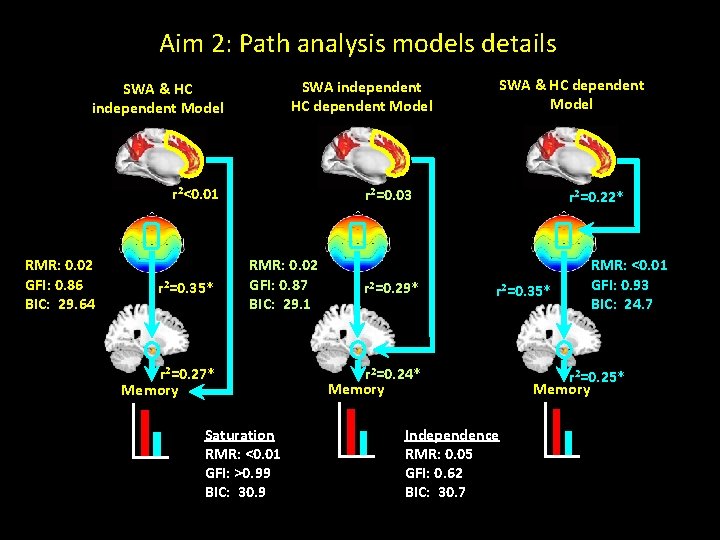
Aim 2: path analysis models SWA & HC independent Model Memory SWA independent HC dependent Model SWA & HC dependent Model Memory m. PFC Aβ influences memory through SWA Memory

Conclusions: Working Model Memory Vicious Cycle

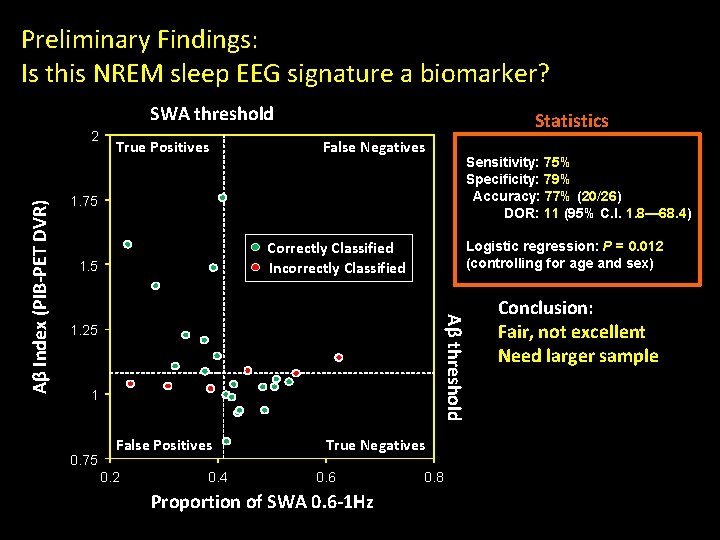
Preliminary Findings: Is this NREM sleep EEG signature a biomarker? SWA threshold True Positives False Negatives Sensitivity: 75% Specificity: 79% Accuracy: 77% (20/26) DOR: 11 (95% C. I. 1. 8— 68. 4) 1. 75 Correctly Classified Incorrectly Classified 1. 5 Logistic regression: P = 0. 012 (controlling for age and sex) Aβ threshold Aβ Index (PIB-PET DVR) 2 Statistics 1. 25 1 0. 75 False Positives 0. 2 0. 4 True Negatives 0. 6 Proportion of SWA 0. 6 -1 Hz 0. 8 Conclusion: Fair, not excellent Need larger sample

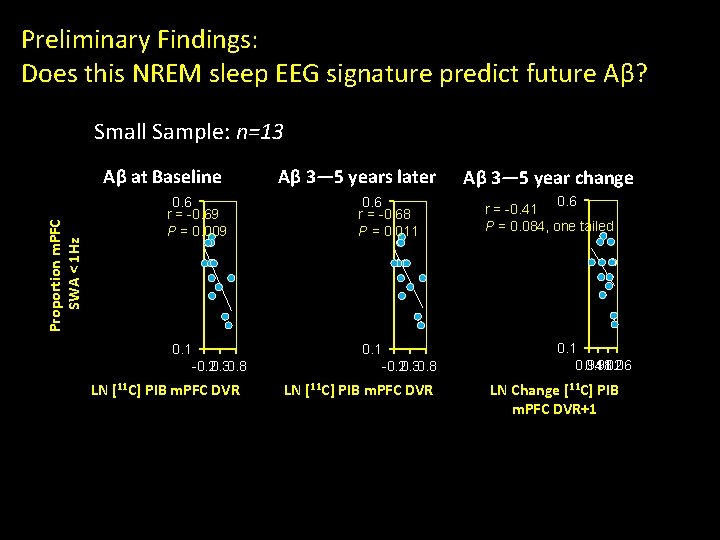
Preliminary Findings: Does this NREM sleep EEG signature predict future Aβ? Small Sample: n=13 Proportion m. PFC SWA < 1 Hz Aβ at Baseline 0. 6 r = -0. 69 P = 0. 009 0. 1 Aβ 3— 5 years later 0. 6 r = -0. 68 P = 0. 011 0. 1 -0. 20. 30. 8 LN [11 C] PIB m. PFC DVR Aβ 3— 5 year change 0. 6 r = -0. 41 P = 0. 084, one tailed 0. 1 0. 94 0. 98 1. 02 1. 06 LN Change [11 C] PIB m. PFC DVR+1

What can you do about it? Recommendations from the National Sleep Foundation • • • If you have sleep problems, talk to your doctor about it! Tell the doctor managing cognitive symptoms about any sleep problems Practice good sleep hygiene as a patient and caregiver – No caffeine after early afternoon, no more than 1 nap (<1 hour) • • Keep a very regular sleep/wake schedule Bright light soon after waking, dim light at night – Use a red nightlight! • Keep simple daily routines to accomplish everyday tasks – Reminder notes help • Create a safe environment – Keep dangerous items out of reach • Stay active, both in mind and body – Exercise at least a little every day – Combine exercise with learning • If you are a caregiver, get help – – Overnight help reduces burden dramatically Get emotional support Have your own social life Get enough rest

What are we doing about it? Experimental methods to enhance slow wave sleep • Pharmacology (Mostly GABAergic) • Transcranial direct current stimulation (t. DCS) • Transcranial Magnetic Stimulation (TMS) • Vestibular stimulation via slow rocking • Acoustic stimulation timed to slow waves

Matt Walker, Ph. D Walkerlab Vikram Rao Andrea Goldstein, Ph. D Stephanie Greer, Ph. D Ingrid Nieuwenhuis, Ph. D Jared Saletin, Ph. D Els van der Helm, Ph. D Adam Krause RAs: David Baquirin Maggie Belshe Meghna Bhatter Michelle Binod Sam Bowditch Catherine Dang Jay Gupta Danny Holzman April Horn Emily Hur Jonathan Jeng Samika Kumar Jack Lindquist Molly Nicholas Sina Rashidi Matthew Shonman Lilly Zhang Alyssa Zhu Acknowledgments Sonia Ancoli-Israel, Ph. D William Jagust, MD Jagustlab Shawn Marks Jacob Vogel Amynta Hayenga Candace Markley Beth Mormino, Ph. D Other collaborators Brandon Lu, MD Adam Gazzaley, MD, Ph. D Michael Rubens Supported by: NIH AG 031164, AG 034570, AG 08415, AG 039170, MH 093537, DA 031939 Sleep Well… !

EXTRA SLIDES

Overall Limitations and next steps: • Cross Sectional Studies – Longitudinal follow-up • Associational Studies – Sleep disruption/enhancement interventions • When successful? When not? • Limited n/Generalizability – Larger follow-up – Multifactorial approach: PET Aβ & τ imaging, APOE genotyping, task & resting-state f. MRI, multiple cognitive domains, VBM, DTI, WMH, etc. – Multiple forms of learning: what about procedural? emotional? – Multiple groups: include middle age group, include cognitive disorder groups, e. g. MCI, early AD, other cognitive disorders like VD, DLB, PD, or FTD?

Sleep in young and older adults

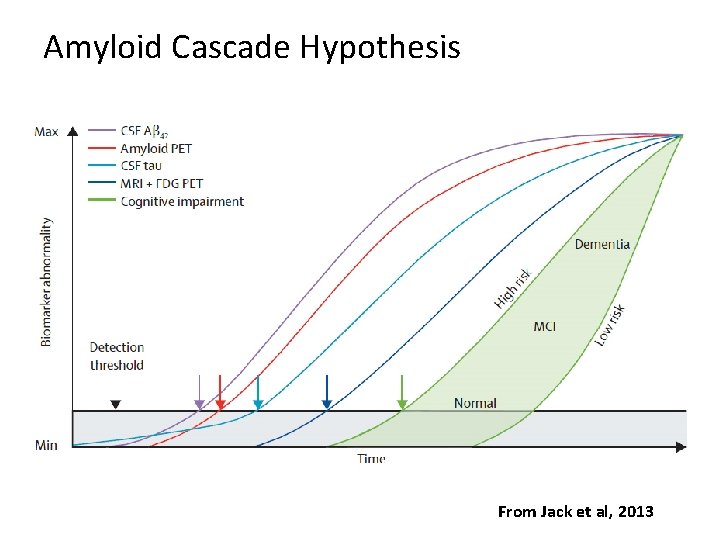
Amyloid Cascade Hypothesis From Jack et al, 2013

Background: β-amyloid pathology Buckner et al, 2005 30% HC adults >65 yo are Aβ+

Extra: Source of NREM SW <1 Hz Source of <1 Hz m. PFC slow waves Min Current density Max CZ and FZ Slow waves <1 Hz sourced to m. PFC

Aim 2: Path analysis models details SWA independent HC dependent Model SWA & HC independent Model r 2<0. 01 RMR: 0. 02 GFI: 0. 86 BIC: 29. 64 r 2=0. 35* SWA & HC dependent Model r 2=0. 03 RMR: 0. 02 GFI: 0. 87 BIC: 29. 1 r 2=0. 27* Memory Saturation RMR: <0. 01 GFI: >0. 99 BIC: 30. 9 r 2=0. 29* r 2=0. 22* r 2=0. 35* r 2=0. 24* Memory Independence RMR: 0. 05 GFI: 0. 62 BIC: 30. 7 RMR: <0. 01 GFI: 0. 93 BIC: 24. 7 r 2=0. 25* Memory

Aim 1 Word-Nonsense Word Task: