Astronomy 101 The Solar System Tuesday Thursday Tom













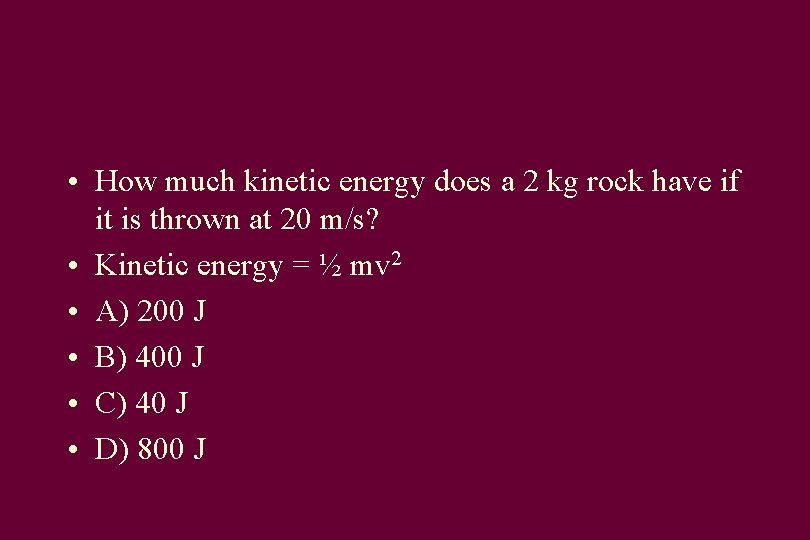
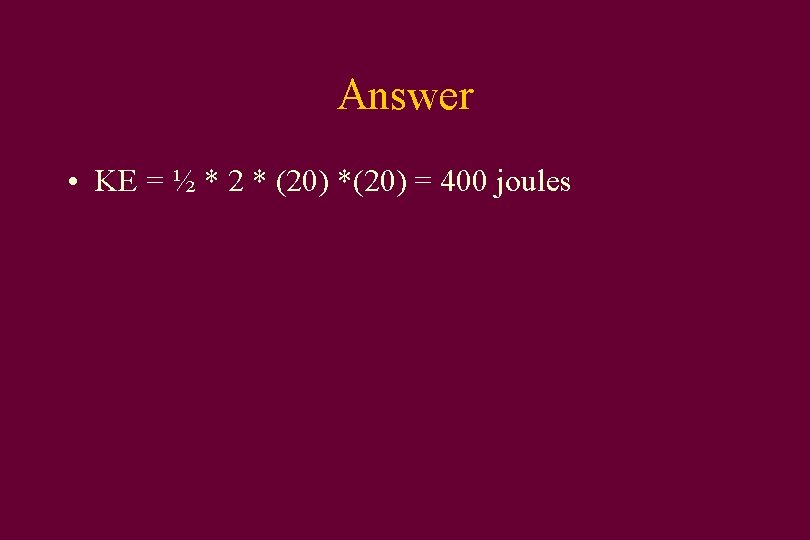









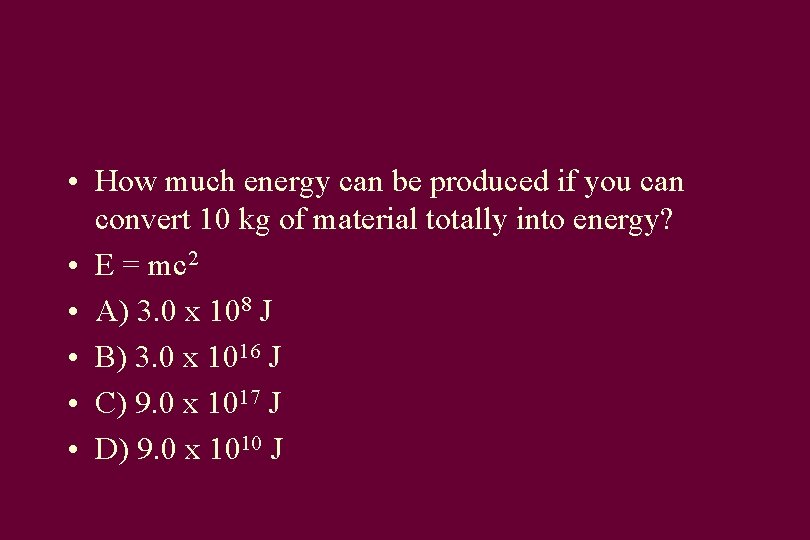
























- Slides: 64

Astronomy 101 The Solar System Tuesday, Thursday Tom Burbine tomburbine@astro. umass. edu

Course • Course Website: – http: //blogs. umass. edu/astron 101 -tburbine/ • Textbook: – Pathways to Astronomy (2 nd Edition) by Stephen Schneider and Thomas Arny. • You also will need a calculator.

• There is an Astronomy Help Desk that is open Monday -Thursday evenings from 7 -9 pm in Hasbrouck 205. • There is an open house at the Observatory every Thursday when it’s clear. Students should check the observatory website before going since the times may change as the semester progresses and the telescope may be down for repairs at times. The website is http: //www. astro. umass. edu/~orchardhill/index. html.

Exam #1 • Average was 85

HW #6 • Due by Feb. 23 rd at 1 pm

In the News: Meteorite • Fell in a doctor’s office in Lorton, Virginia

In the News: Asteroid Collision http: //www. csmonitor. com/Science/Discoveries/2010/0202/Has-the-Hubble-Space-Telescope-spied-asteroid-on-asteroid-collision-debris

Energy • Energy is the ability to generate motion

Conservation of Energy • Energy is neither created or destroyed – it just changes forms • Conservation of Energy – The energy in a closed system may change form, but the total amount of energy does not change as a result of any process.

Energy units • In English Units, we use calories to measure energy • In science (and in this class), we will use joules to measure energy • 1 Joule = 1 kg*m 2/s 2

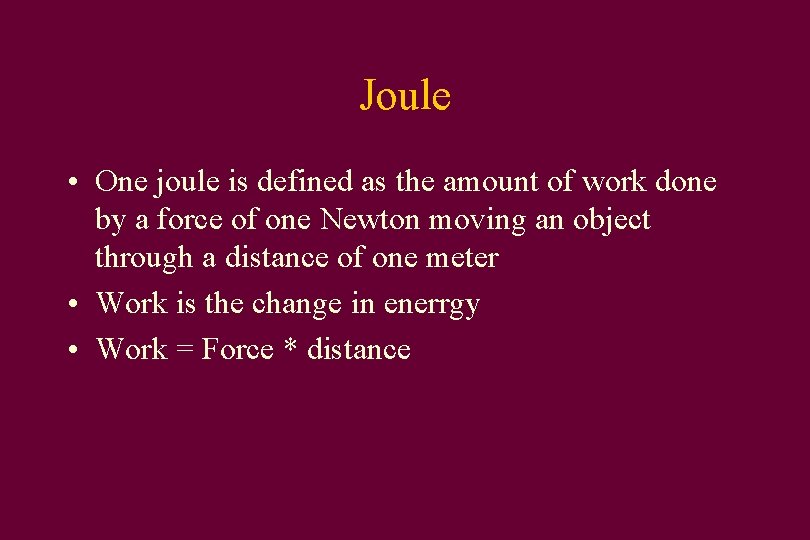
Joule • One joule is defined as the amount of work done by a force of one Newton moving an object through a distance of one meter • Work is the change in enerrgy • Work = Force * distance


3 basic categories of energy • Kinetic energy – energy of motion • Potential energy – energy being stored for possible conversion into kinetic energy • Radiative energy – energy carried by light

Kinetic energy • • Kinetic energy = ½ mv 2 m is mass in kg v is velocity in meters/s Remember: a joule has units of kg*m 2/s 2
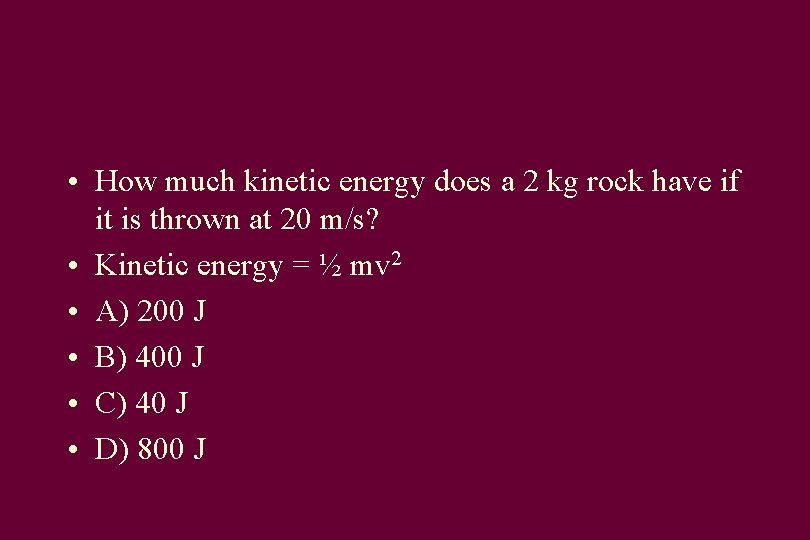
• How much kinetic energy does a 2 kg rock have if it is thrown at 20 m/s? • Kinetic energy = ½ mv 2 • A) 200 J • B) 400 J • C) 40 J • D) 800 J
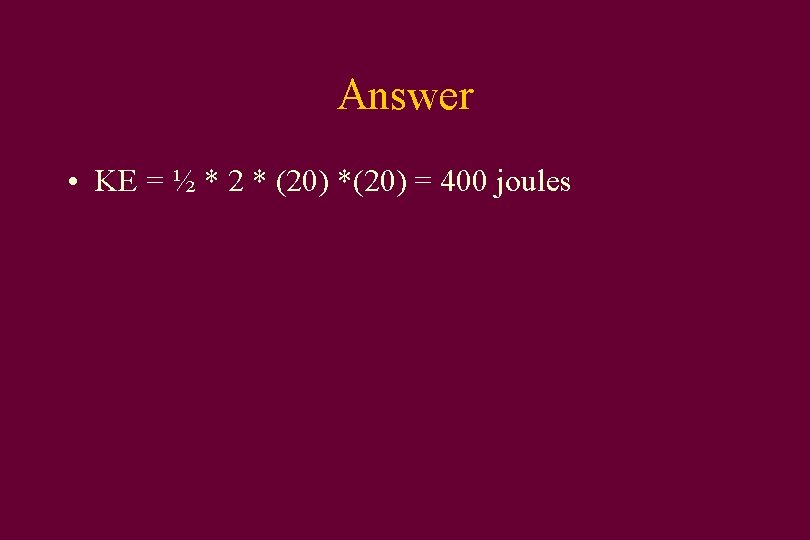
Answer • KE = ½ * 2 * (20) *(20) = 400 joules

Thermal energy (kind of kinetic energy) • Temperature is a measure of the average kinetic energy of the particles • Higher temperature – more kinetic energy, particles moving faster • For examples, air molecules around you are moving at ~600 m/s http: //eo. ucar. edu/webweather/molecules. html

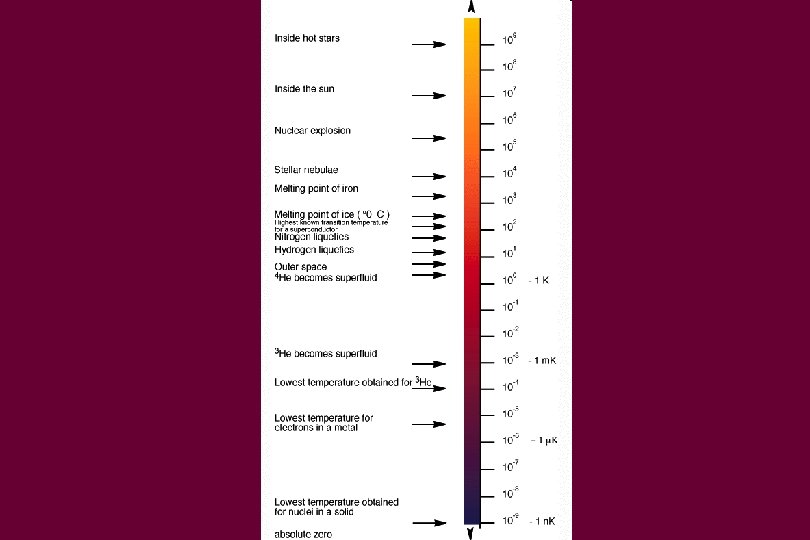
Temperature scales • • • In America, we use Fahrenheit Water freezes at 32 degrees F Water boils at 212 degrees F Everywhere else, they use Celsius Water freezes at 0 degrees C Water boils at 100 degrees C

In Science • Temperature is measured in Kelvin • Zero Kelvin is absolute zero – nothing moves • Add 273. 15 to the Celsius temperature to get the Kelvin temperature • 273. 15 Kelvin = 0 degrees Celsius



Gravitational Potential Energy • Gravitational Potential Energy released as an object falls depends on its mass, the strength of gravity, and the distance it falls • For example, your gravitational potential energy increases as you go farther up in the air • This is because you hit the ground at a faster speed if you jump from a higher distance

• KE + PE = 0 • As kinetic energy increases, potential energy decreases

Converting Mass to Energy • What is the most famous formula in the world?

E = mc 2 • • m is mass in kilograms c is speed of light in meters/s (3 x 108 m/s) So E is in joules very small amounts of mass may be converted into a very large amount of energy and

Who came up with it?

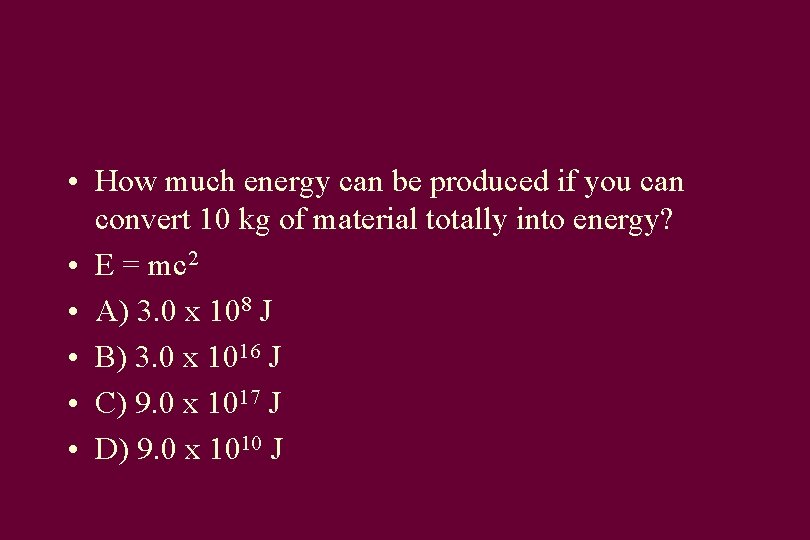
• How much energy can be produced if you can convert 10 kg of material totally into energy? • E = mc 2 • A) 3. 0 x 108 J • B) 3. 0 x 1016 J • C) 9. 0 x 1017 J • D) 9. 0 x 1010 J

Answer • • E = 10 kg * (3 x 108 m/s) E = 10* (9 x 1016) J E = 90 x 1016 J E = 9. 0 x 1017 J

Mass-Energy • E=mc 2 • So Mass is a form of potential energy • Where is one place where you see mass converted into energy?


Light • Light is a form of energy

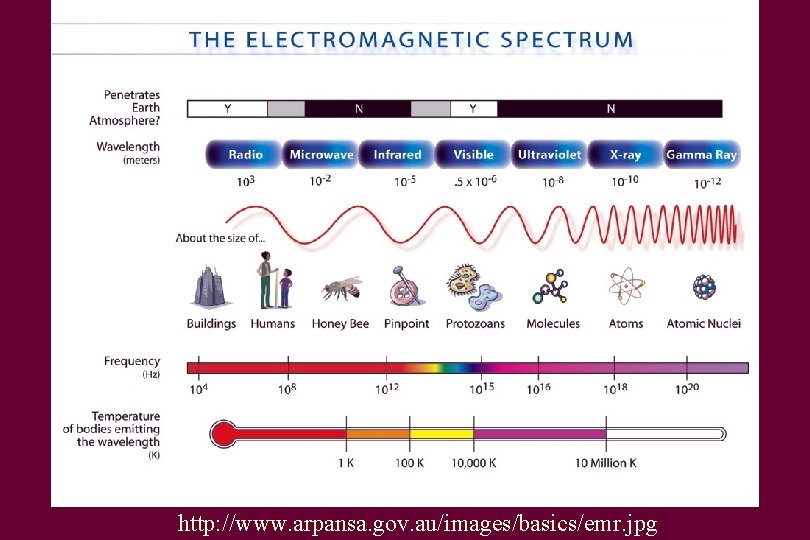
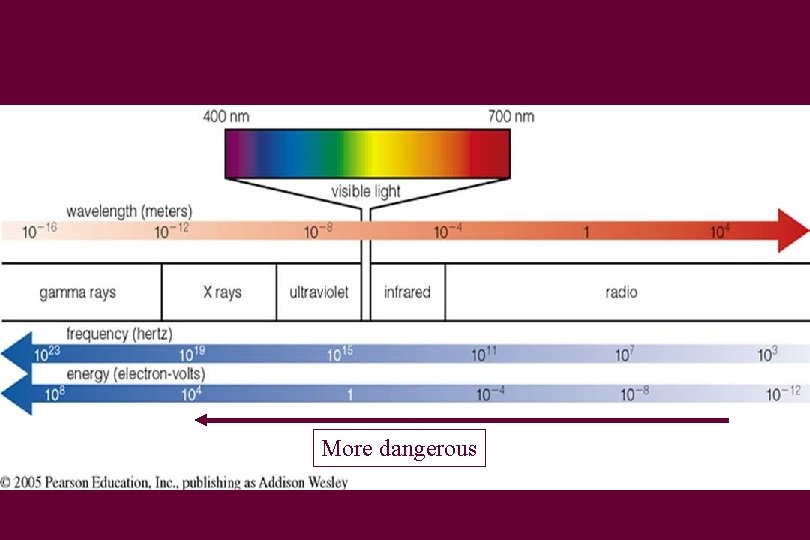
Light • These are all forms of light – Gamma rays – X-rays – Ultraviolet light – Visible light – Infrared light – Radio waves

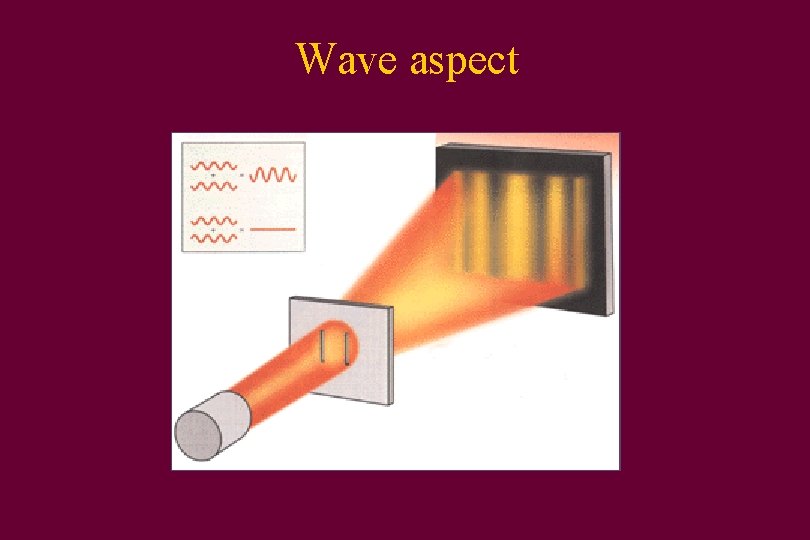
Light • Can act as a particle • Can also act as a wave

Particle aspect • Particles called photons stream from the Sun and can be blocked by your body

Photons • Light is quantized • Comes in discrete packets called photons

Wave aspect

Thomas Young Experiment • http: //micro. magnet. fsu. edu/primer/java/interference/dou bleslit/

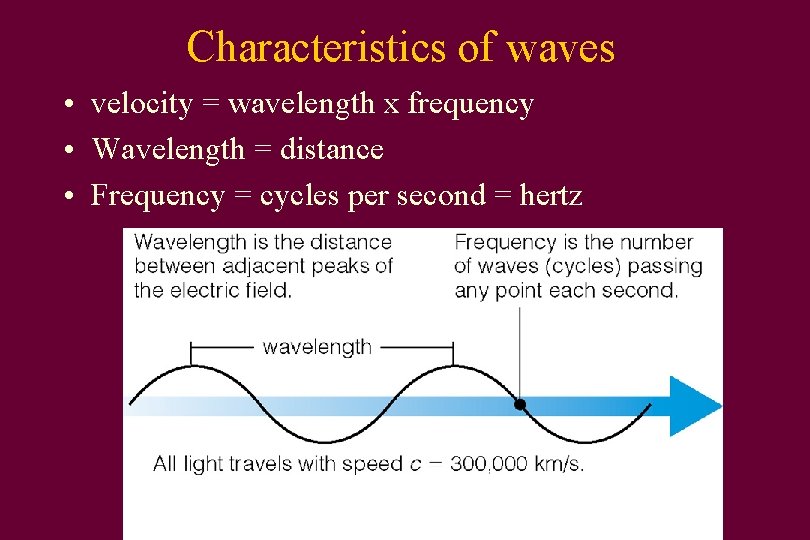
Characteristics of waves • velocity = wavelength x frequency • Wavelength = distance • Frequency = cycles per second = hertz

For light • • c = wavelength x frequency In vacuum, speed of light stays the same So if wavelength goes up Frequency does down f = frequency λ = wavelength c=λxf

Calculations • • • c=λxf So if the wavelength is 1 x 10 -12 m 3 x 108 m/s = 1 x 10 -12 m * f f = 3 x 108 m/s/1 x 10 -12 m f = 3 x 1020 s-1 = 3 x 1020 Hz

Calculations • • • c=λxf So if the frequency is 1 x 1015 Hz 3 x 108 m/s = λ * 1 x 1015 Hz λ = 3 x 108 m/s/1 x 1015 Hz λ = 3 x 10 -7 m

Energy of light • • • Energy is directly proportional to the frequency E=h*f h = Planck’s constant = 6. 626 x 10 -34 J*s since f = c/λ Energy is inversely proportional to the wavelength E = hc/λ

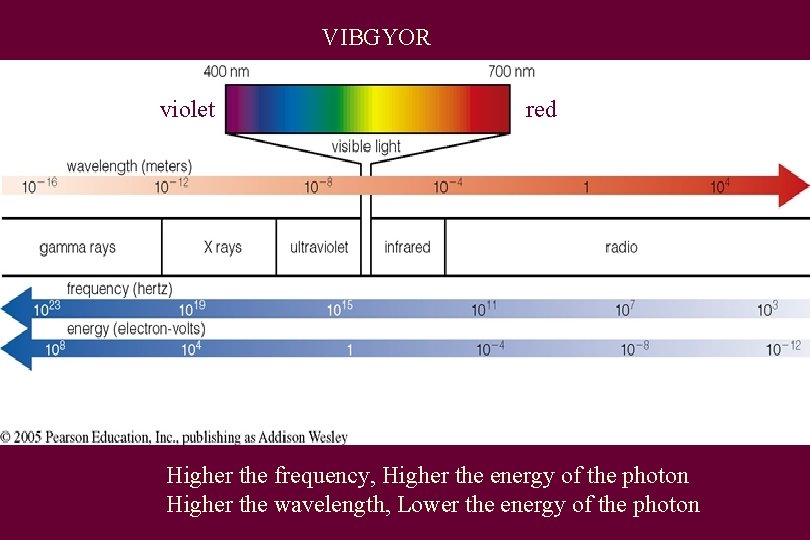
VIBGYOR violet red Higher the frequency, Higher the energy of the photon Higher the wavelength, Lower the energy of the photon

ROYGBIV • Red – long wavelength • Violet – short wavelength

http: //www. arpansa. gov. au/images/basics/emr. jpg

Calculations • What is the energy of a radio wave with a frequency of 1 x 107 Hz? • E=h*f • h = Planck’s constant = 6. 626 x 10 -34 J*s • E = 6. 626 x 10 -34 J*s * 1 x 107 • E = 6. 626 x 10 -27 J

Calculations • What is the energy of a gamma ray photon with wavelength of 1 x 10 -15 m • E = hc/λ • h = Planck’s constant = 6. 626 x 10 -34 J*s • E = 6. 626 x 10 -34 J*s * 3 x 108 m/s / 1 x 10 -15 m • E = 1. 99 x 10 -10 J

So why are some types of radiation dangerous? • Higher the energy, the farther the photons can penetrate • So gamma and X-rays can pass much more easily into your the body • These high-energy photons can ionize atoms in cells • Ionization means removes electrons from an atom

More dangerous

When you measure an astronomical body • You measure intensity • Intensity – amount of radiation

Matter • Matter is material

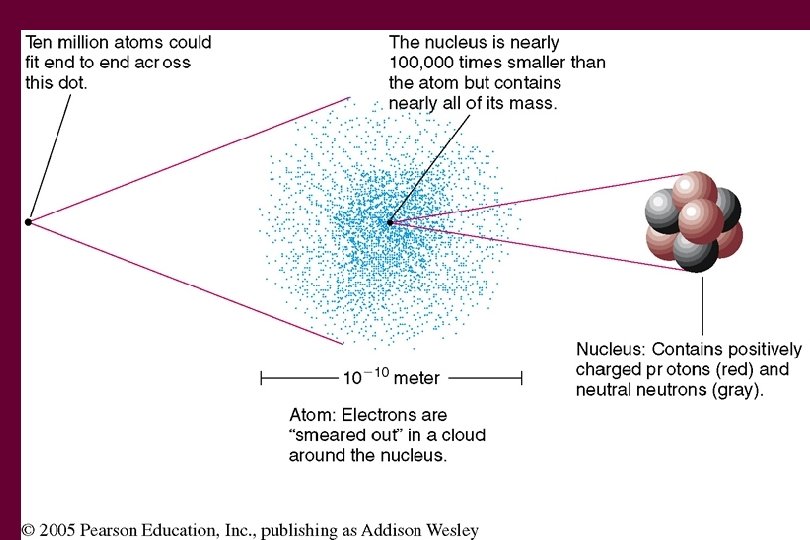
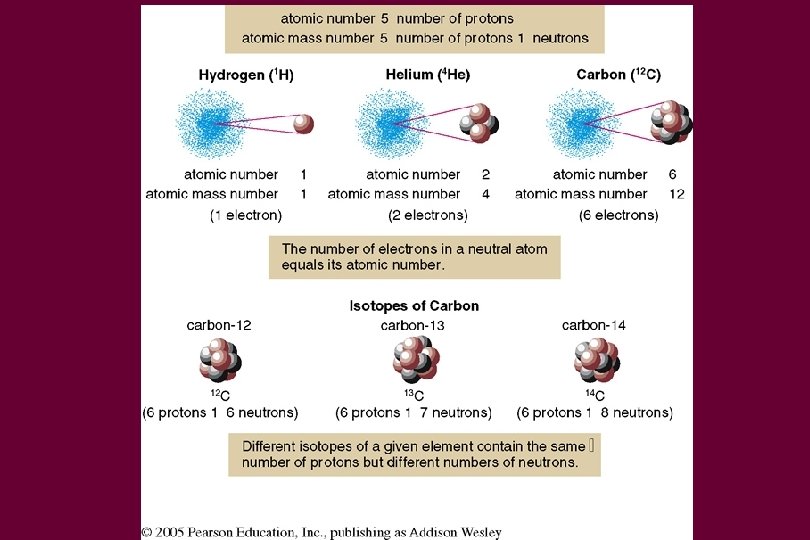
Atoms • • • Atoms are made up of 3 types of particles Protons – positive charge (+1) Electrons – negative charge (-1) Neutrons – neutral charge (no charge) Protons and Neutrons are found in the nucleus



Elements • http: //www. periodictable. com/

Elements • Different elements have different numbers of protons • The properties of an atom are a function of the electrical charge of its nucleus

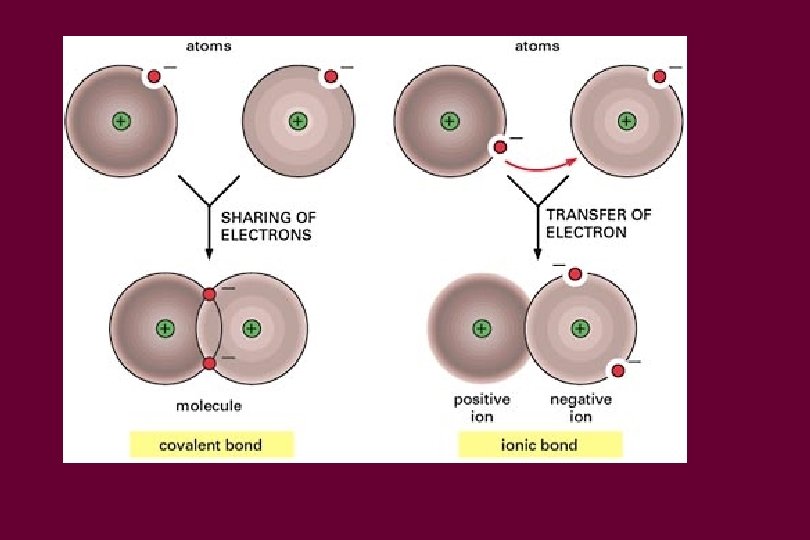
Charge • If an atom has the same number of electrons and protons, it has a neutral charge • More electrons than protons, negatively charged • More protons than electrons, positive charged • Neutrons have neutral charge so don’t affect the charge of an atom


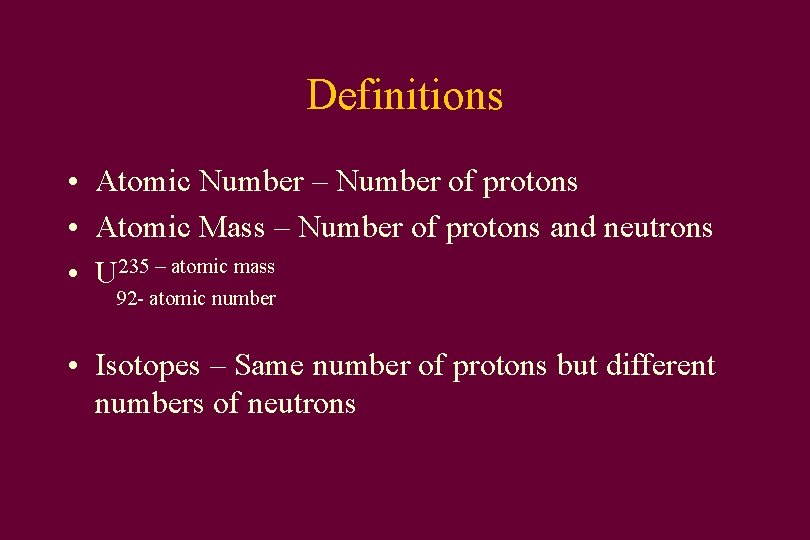
Definitions • Atomic Number – Number of protons • Atomic Mass – Number of protons and neutrons • U 235 – atomic mass 92 - atomic number • Isotopes – Same number of protons but different numbers of neutrons

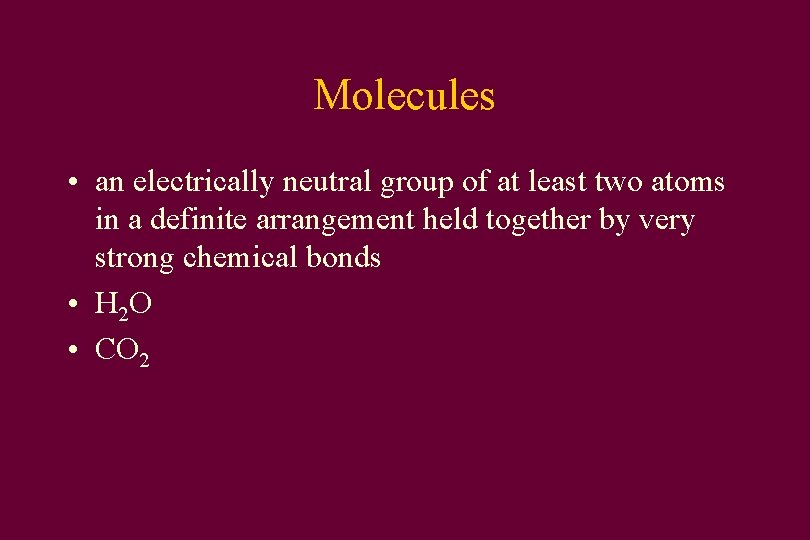
Molecules • an electrically neutral group of at least two atoms in a definite arrangement held together by very strong chemical bonds • H 2 O • CO 2


• http: //www. hulu. com/watch/63320/cosmos-thelives-of-the-stars

Any Questions?
 Monday tuesday wednesday thursday friday calendar
Monday tuesday wednesday thursday friday calendar Monday tuesday wednesday thursday friday saturday sunday
Monday tuesday wednesday thursday friday saturday sunday Monday inglês
Monday inglês Learning astronomy by doing astronomy activity 1 answers
Learning astronomy by doing astronomy activity 1 answers Learning astronomy by doing astronomy
Learning astronomy by doing astronomy Learning astronomy by doing astronomy activity 1 answers
Learning astronomy by doing astronomy activity 1 answers Solar system astronomy class
Solar system astronomy class Solar system astronomy class
Solar system astronomy class Solar system astronomy class
Solar system astronomy class Astronomy 101 formulas
Astronomy 101 formulas Go 910
Go 910 What does tom symbolize in the devil and tom walker
What does tom symbolize in the devil and tom walker Wholesale solar power panel
Wholesale solar power panel An inexhaustible source of energy
An inexhaustible source of energy Thoughtful thursday morning message
Thoughtful thursday morning message St paul's church wibsey
St paul's church wibsey Holy thursday (songs of innocence)
Holy thursday (songs of innocence) Blessed holy thursday
Blessed holy thursday 997ni1xcmkw -site:youtube.com
997ni1xcmkw -site:youtube.com How's the weather there answer
How's the weather there answer Terrific thursday
Terrific thursday Thursday bellwork
Thursday bellwork Happy easter traditions
Happy easter traditions Fright night: resurrection
Fright night: resurrection Thursday question of the day
Thursday question of the day Thursday bell ringer
Thursday bell ringer Thursday prayer images
Thursday prayer images Thursday prayer
Thursday prayer Thursday bell ringers
Thursday bell ringers Wednesday bellringer
Wednesday bellringer Welcome thursday
Welcome thursday Eugene kelly colgate
Eugene kelly colgate My favourite subject maths for class 4
My favourite subject maths for class 4 Adjectives for tuesday
Adjectives for tuesday Oeoes uc davis
Oeoes uc davis Thursday420
Thursday420 Imam zain ul abideen dua
Imam zain ul abideen dua Romeo and juliet time line
Romeo and juliet time line Senior g friendship thursday
Senior g friendship thursday Welcome thursday
Welcome thursday New deal tennessee valley authority
New deal tennessee valley authority Thursday
Thursday Sunday monday tuesday wednesday
Sunday monday tuesday wednesday Thursday morning prayer
Thursday morning prayer On thursday we visited
On thursday we visited This coming thursday
This coming thursday Monday through thursday
Monday through thursday Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worms-breton
Tư thế worms-breton Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng tiếng nhảy
Các môn thể thao bắt đầu bằng tiếng nhảy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra