ASTM D 6422 whats happening Determining water tolerance







- Slides: 14

ASTM D 6422 – what’s happening? Determining water tolerance of gasoline-ethanol-water mixtures

Outline • Phase equilibria in ethanol-Gasoline blends • Phase stability in Hydrous Ethanol-Gasoline mixtures • Suggestions for improvement © 2007 Process Design Center

Introductory remarks • Let’s focus on ethanol • Analysis by simulation of phase equilibria • Ternary mixtures • Gasoline – water - ethanol • Benzene – water – ethanol • Hexane – water - ethanol • Gasoline as single component • All calculations done at 68 °F (20 °C) • Experimental work • Observations at TU Delft • Standard tests at SGS (independent test laboratory) © 2007 Process Design Center

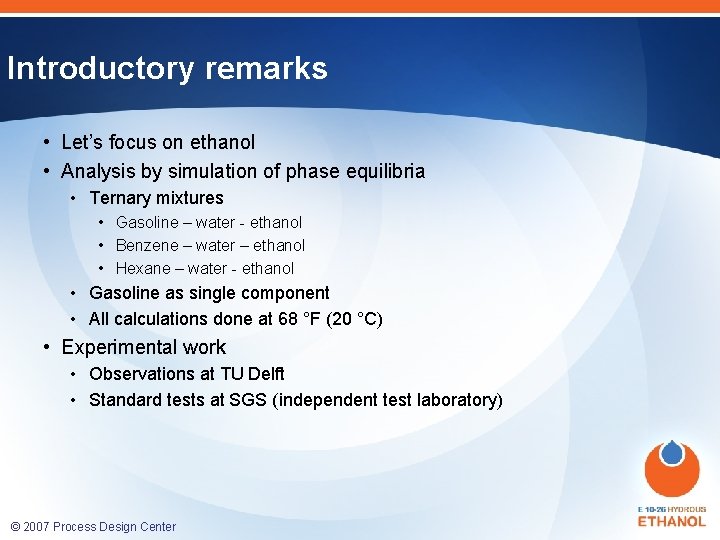
Ternary phase diagram: introduction ETHANOL 20% ha Et 40% W 40% 60% 20% GASOLINE 80% 60% 40% Weight Percent Gasoline © 2007 Process Design Center er at eig W ht nt ce Pe rce er nt 60% P ht eig W no l 80% 20% WATER

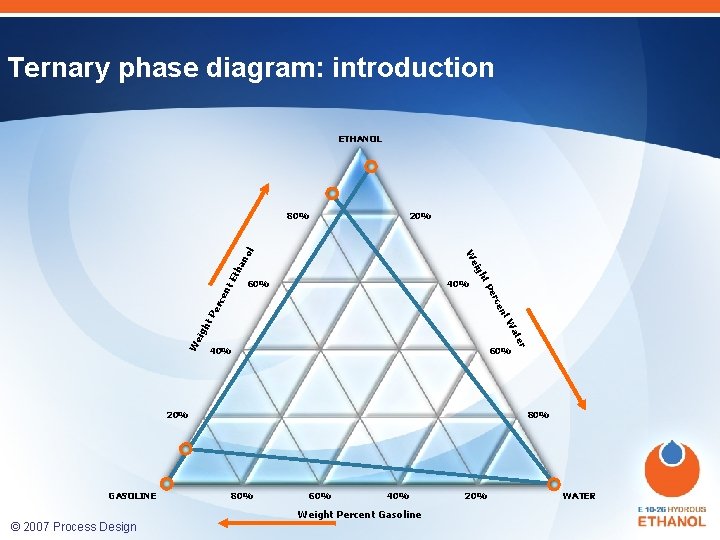
Phase behavior of gasoline-ethanol-water mixtures ETHANOL 80% 20% ha Et W 40% 60% 20% GASOLINE 80% 60% 40% Weight Percent Gasoline © 2007 Process Design Center er at eig ht W nt Pe rce 40% rce Pe 60% ht nt • density: how well it separates into lower - and upper layer • surface tension: drop coalescence • refractive index: observability eig W no l Determining The location of binodal reasons water forthe thistolerance are: curve involves incipient depends on: energies • Lowdetecting free of mixing phase separation. Inphases the region • gasoline composition Meta-stability of indicated thisproperties isofvery to • presence otherdifficult Similar of observe and easily leads to oxygenates phases false • conclusions. temperature 20% WATER

Experimental work • It has been experimentally verified that the phase boundary cannot be well observed by visual inspection. ethanol gasoline • In this example the mixture was well in the two-phase region before haziness persisted and a second liquid layer started to settle. After several hours, the phases were still not clear and the compositions (see graph) were not at equilibrium. © 2007 Process Design Center water

When does phase separation occur ? • Free Energy: the arbiter for change • A spontaneous change will only occur if heat can be released to the environment • The Free Energy is the heat that a changing system releases into the environment • A system changes until it obtains minimum Free Energy • However there may be obstacles for spontaneous change to take place © 2007 Process Design Center

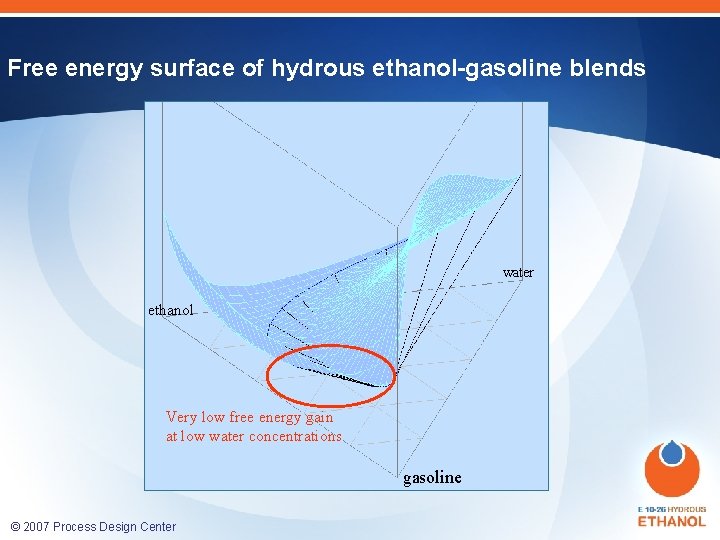
Free energy surface of hydrous ethanol-gasoline blends Large free energy gain at low ethanol content ethanol water ethanol Very low free energy gain at low water concentrations gasoline © 2007 Process Design Center gasoline water

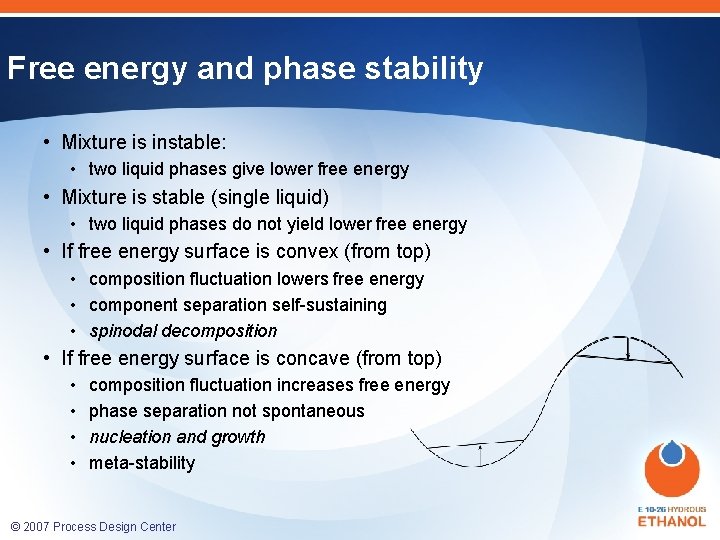
Free energy and phase stability • Mixture is instable: • two liquid phases give lower free energy • Mixture is stable (single liquid) • two liquid phases do not yield lower free energy • If free energy surface is convex (from top) • composition fluctuation lowers free energy • component separation self-sustaining • spinodal decomposition • If free energy surface is concave (from top) • • composition fluctuation increases free energy phase separation not spontaneous nucleation and growth meta-stability © 2007 Process Design Center

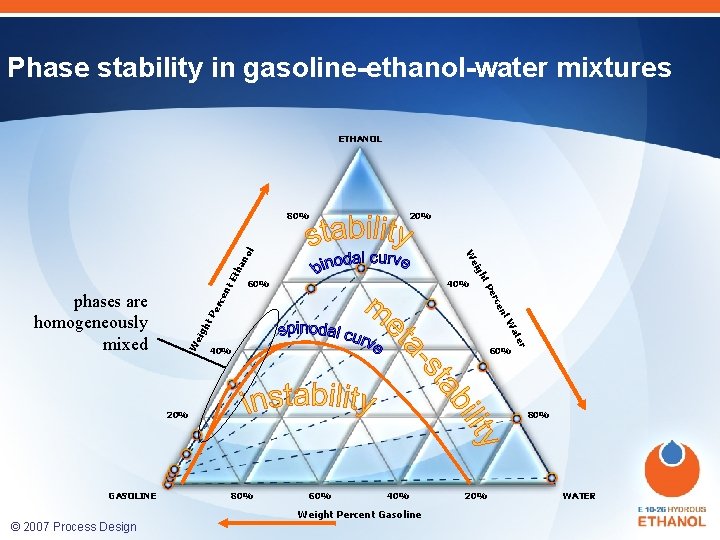
Phase stability in gasoline-ethanol-water mixtures ETHANOL 20% ha Et nt 40% W 40% 60% 20% GASOLINE 80% 60% 40% Weight Percent Gasoline © 2007 Process Design Center er at eig W ht nt ce Pe rce er phases are homogeneously mixed 60% P ht eig W no l 80% 20% WATER

Spontaneity of phase change • Free energy of phase change (instability) • Free energy curvature of phase change (meta-stability) • Creation of interfacial area • work required due to surface tension > free energy of phase change • phase separation may be delayed or prevented • Other obstacles • diffusion: low concentration gradients, slow mass transport • structured solutions (strong hydrogen bonds at low temperatures) (energy of dissociation > free energy of phase change) • Composition has large effect on binodal & spinodal curve • paraffinic - aromatic content • presence of other oxygenates • ethers, higher alcohols • co-solvency © 2007 Process Design Center

Consequences for the test method • Since: • • • visual observation of phase separation can be difficult phase separation may be delayed or even prevented changes occur gradually with temperature phase homogeneity plays an important role the mixtures may be rather hygroscopic • a good test method requires: • advanced detection of phase behavior • good mixing • no cylindrical vessel (bad axial mixing) • controlled stirring • no “dead” zones • minimize water uptake (at low temperatures) • re-define test method’s objective (performance test) © 2007 Process Design Center

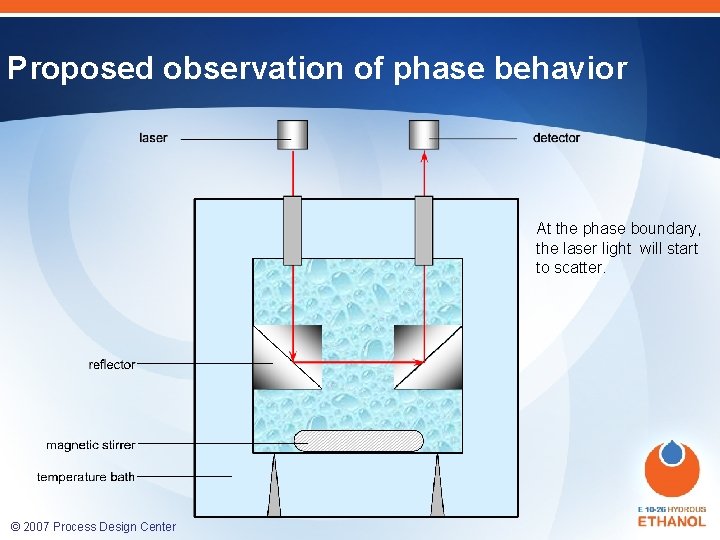
Proposed observation of phase behavior At the phase boundary, the laser light will start to scatter. © 2007 Process Design Center

HE Blends B. V. Catharinastraat 21 f P. O. Box 7052 NL-4800 GB Breda The Netherlands www. e 15 blends. com Process Design Center B. V. tel: +31 76 530 1900 www. process-design-center. com fax: +31 76 522 5934