Asthma Treatment in Young Children Insights from NIH







![FEV 1 After Bronchodilator CAMP, NEJM 343(15) 2000. Copyright © [2000] Massachusetts Medical Society. FEV 1 After Bronchodilator CAMP, NEJM 343(15) 2000. Copyright © [2000] Massachusetts Medical Society.](https://slidetodoc.com/presentation_image_h/82a0f46b5fa9fcfeceb0c28b14089abf/image-11.jpg)


















- Slides: 42

Asthma Treatment in Young Children: Insights from NIH Asthma Network Studies Stanley J. Szefler, MD Helen Wohlberg & Herman Lambert Chair in Pharmacokinetics, National Jewish Health & Professor of Pediatrics and Pharmacology, University of Colorado School of Medicine

Overview • To review the approach to managing wheezing episodes in young children. • To discuss the benefits and limitations of inhaled corticosteroids for managing early asthma. • To summarize new studies directed to improving the management of wheezing episodes in young children.

Disclosure Consultant for Boehringer Ingelheim, Genentech, Glaxo Smith Kline, Merck, Novartis Grant support: NHLBI Childhood Asthma Management Program, Asthma Clinical Research Network, Childhood Asthma Research and Education Network and Asthma. Net NIAID Inner City Asthma Consortium NIEHS/EPA Childhood and Environmental Health Center Grant

Disclosure Grant support (continued): CDPHE Colorado Cardiovascular, Cancer and Pulmonary Disease Program Caring for Colorado Foundation, Glaxo Smith Kline Health Outcomes Program.

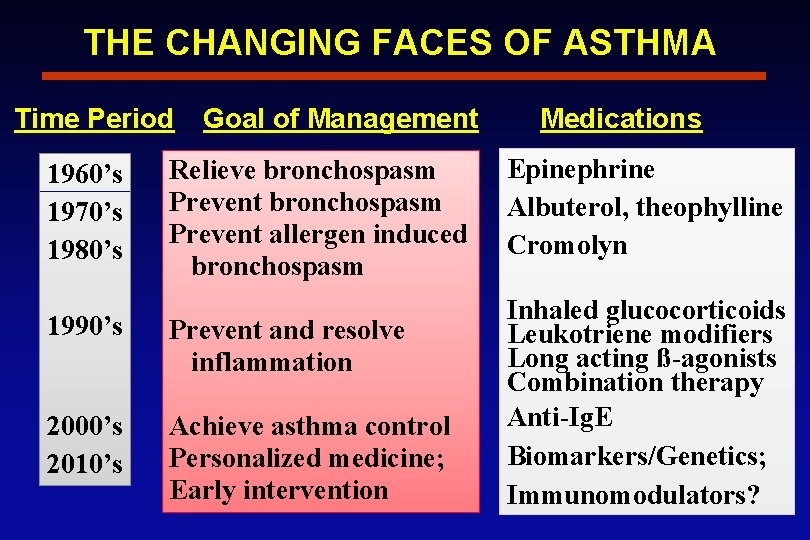
THE CHANGING FACES OF ASTHMA Time Period 1960’s 1970’s 1980’s Goal of Management Relieve bronchospasm Prevent allergen induced bronchospasm 1990’s Prevent and resolve inflammation 2000’s 2010’s Achieve asthma control Personalized medicine; Early intervention Medications Epinephrine Albuterol, theophylline Cromolyn Inhaled glucocorticoids Leukotriene modifiers Long acting ß-agonists Combination therapy Anti-Ig. E Biomarkers/Genetics; Immunomodulators?

Improving Asthma Control Goals of long-term control therapy • Prevent symptoms • Improve pulmonary function • Reduce inflammation • Resolve and prevent progression

Early Intervention for Asthma Prevention Environment • Allergens • Microbes • Pollutants • Stress Biological & Genetic Risk Age • Immune • Lung • Repair 1 o Prevention Aberrant immune development & responses • Viral infections Airways • Aeroallergens Atopy Injury • Pollutants/toxicants Aberrant Repair • Persistent inflammation • BHR • Tissue remodeling • Lung growth/differentiation ASTHMA 2 o Prevention Early Intervention - Liu AH. JACI, 113: S 19024, 2004.

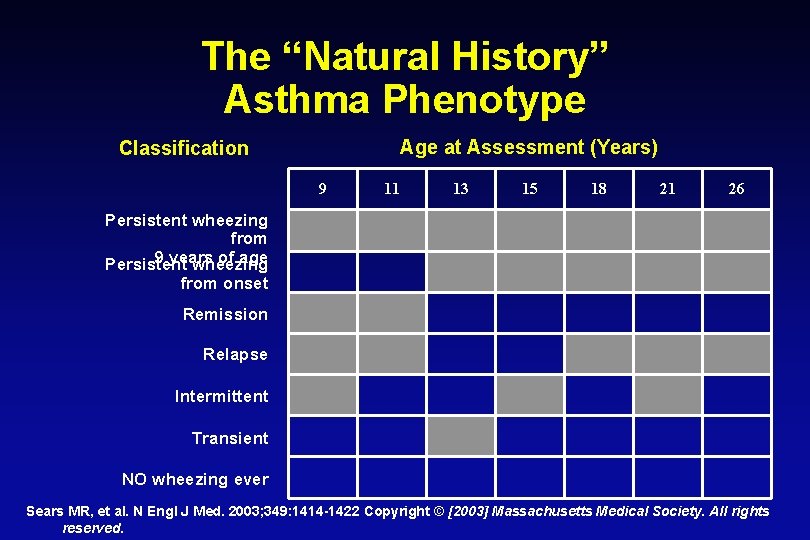
The “Natural History” Asthma Phenotype Age at Assessment (Years) Classification 9 11 13 15 18 21 26 Persistent wheezing from 9 years of age Persistent wheezing from onset Remission Relapse Intermittent Transient NO wheezing ever Sears MR, et al. N Engl J Med. 2003; 349: 1414 -1422 Copyright © [2003] Massachusetts Medical Society. All rights reserved.

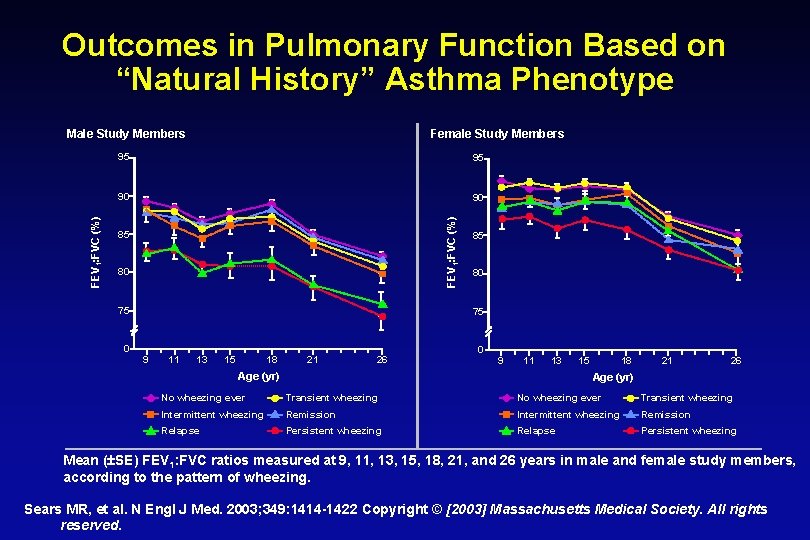
Outcomes in Pulmonary Function Based on “Natural History” Asthma Phenotype Female Study Members 95 95 90 90 FEV 1: FVC (%) Male Study Members 85 80 75 0 85 80 75 9 11 13 15 18 21 26 0 9 11 13 Age (yr) 15 18 21 26 Age (yr) No wheezing ever Transient wheezing Intermittent wheezing Remission Relapse Persistent wheezing Mean ( SE) FEV 1: FVC ratios measured at 9, 11, 13, 15, 18, 21, and 26 years in male and female study members, according to the pattern of wheezing. Sears MR, et al. N Engl J Med. 2003; 349: 1414 -1422 Copyright © [2003] Massachusetts Medical Society. All rights reserved.

Childhood Asthma Management Program Clinical measures of control strongly favored Budesonide over Placebo - Symptoms - Rescue medication use and prednisone courses - Episode-free days - Hospitalizations and urgent care - Initiation of beclomethasone or additional asthma medication CAMP, NEJM 343(15) 2000
![FEV 1 After Bronchodilator CAMP NEJM 34315 2000 Copyright 2000 Massachusetts Medical Society FEV 1 After Bronchodilator CAMP, NEJM 343(15) 2000. Copyright © [2000] Massachusetts Medical Society.](https://slidetodoc.com/presentation_image_h/82a0f46b5fa9fcfeceb0c28b14089abf/image-11.jpg)
FEV 1 After Bronchodilator CAMP, NEJM 343(15) 2000. Copyright © [2000] Massachusetts Medical Society. All rights reserved.

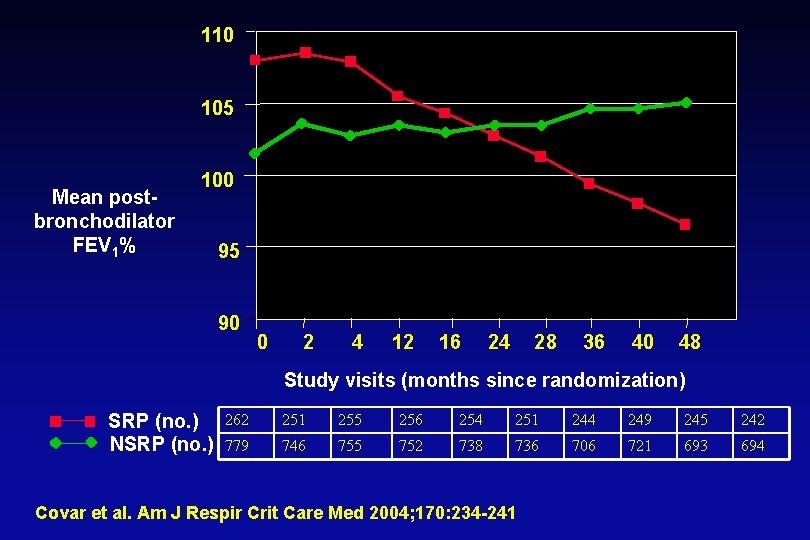
110 105 Mean postbronchodilator FEV 1% 100 95 90 0 2 4 12 16 24 28 36 40 48 Study visits (months since randomization) SRP (no. ) NSRP (no. ) 262 251 255 256 254 251 244 249 245 242 779 746 755 752 738 736 706 721 693 694 Covar et al. Am J Respir Crit Care Med 2004; 170: 234 -241

Potential Approaches to Improving Asthma Control in Young Children • Early intervention • • Combination therapy Biomarkers Genetics Immunomodulators

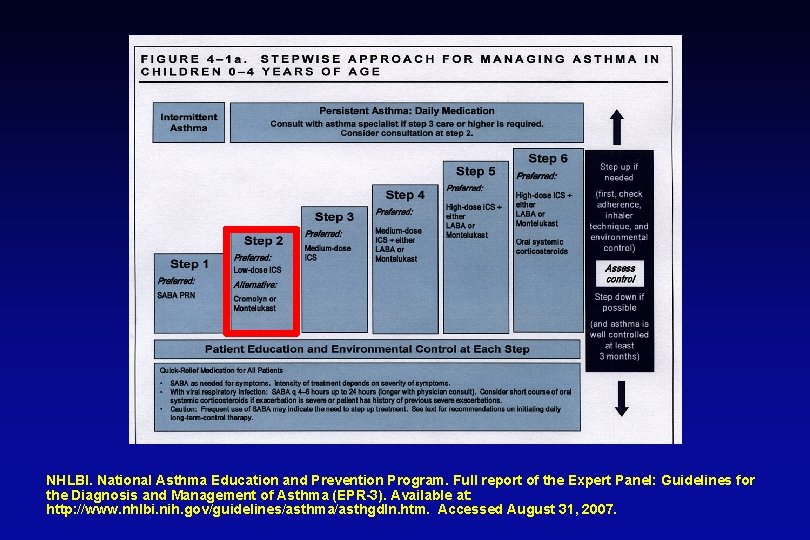
Key Recommendations Managing asthma in children 0 - 4 years • Diagnosis is often difficult. • Treatment has not been adequately studied. • Criteria for initiation of long-term control therapy: - 3 wheezing episodes in past year and positive asthma risk profile. - those who require symptomatic treatment > 2 days per week - two or more severe exacerbations within 6 months

NHLBI. National Asthma Education and Prevention Program. Full report of the Expert Panel: Guidelines for the Diagnosis and Management of Asthma (EPR-3). Available at: http: //www. nhlbi. nih. gov/guidelines/asthma/asthgdln. htm. Accessed August 31, 2007.

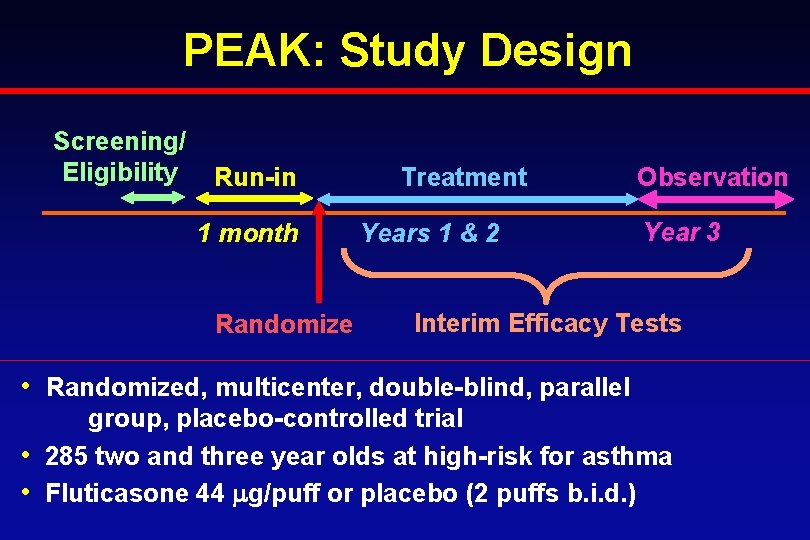
PEAK: Study Design Screening/ Eligibility Run-in 1 month Randomize Treatment Years 1 & 2 Observation Year 3 Interim Efficacy Tests • Randomized, multicenter, double-blind, parallel group, placebo-controlled trial • 285 two and three year olds at high-risk for asthma • Fluticasone 44 g/puff or placebo (2 puffs b. i. d. )

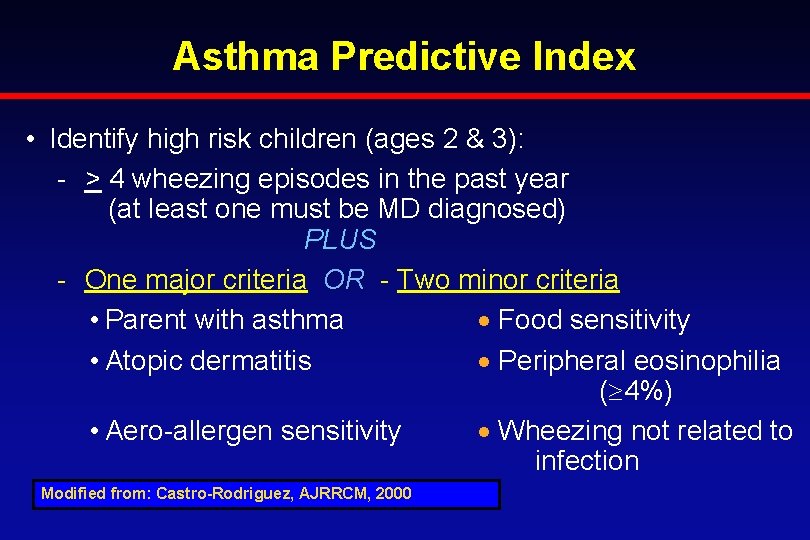
Asthma Predictive Index • Identify high risk children (ages 2 & 3): - > 4 wheezing episodes in the past year (at least one must be MD diagnosed) PLUS - One major criteria OR - Two minor criteria • Parent with asthma Food sensitivity • Atopic dermatitis Peripheral eosinophilia ( 4%) • Aero-allergen sensitivity Wheezing not related to infection Modified from: Castro-Rodriguez, AJRRCM, 2000

PEAK Study: Episode-free Days Treatment Guilbert TW and CARE Network. NEJM 2006; 354: 1985 -97. Observation

Characteristics Associated with EFD Response Percentage of EFDs Stratifying Variable ICS Mean (95% CI) Placebo Mean (95% CI) Difference (95% CI) P-value (ICS vs Placebo) Male 93 (92, 95) 86 (83, 89) 7. 3 (3. 9, 11. 1) 0. 0005 Female 92 (89, 94) 0. 1 (-3. 4, 3. 5) 0. 9 Caucasian 93 (91, 95) 84 (80, 88) 9. 1 (4. 8, 13. 9) 0. 0001 Non-Caucasian 92 (89, 94) 93 (91, 94) -1. 0 (-3. 9, 1. 7) 0. 6 Run-In EFD <80% 92 (90, 94) 84 (79, 87) 8. 6 (4. 2, 13. 2) 0. 0009 Run-In EFD >=80% 93 (91, 95) 0. 0 (-2. 5, 2. 5) 0. 9 ED/Hospitalization History 95 (93, 96) 87 (83, 90) 7. 7 (3. 9, 11. 6 0. 0004 No ED/Hospitalization History 90 (87, 92) 91 (89, 93) -1. 1 (-4. 4, 2. 1) 0. 6 ≥ 1 Positive Aeroallergen Skin Test 93 (91, 94) 86 (83, 89) 6. 5 (3. 2, 10. 0) 0. 0027 Negative Aeroallergen Skin Test 93 (90, 95) 92 (89, 94) 0. 9 (-2. 5, 4. 4) 0. 6 Bacharier LB et al. J Allergy Clin Immunol 2009

Acute Intervention Management Strategies (AIMS) A study evaluating the effects of episodic use of an inhaled corticosteroid or a leukotriene receptor antagonist on the frequency and severity of acute wheezing episodes in young children

Background • Population of interest - Young children with intermittent wheezing and severe exacerbations • Research questions - Does treatment with ICS or LTRA early in the course of acute respiratory tract illnesses increase the proportion of episode free days over a 12 month period compared to conventional therapy*? - Do these treatments modify the severity of the acute episodes? *Conventional therapy - inhaled bronchodilator followed by the sequential addition of systemic corticosteroids

Inclusion Criteria 1. Age 12 -59 months 2. Recurrent episodes ( 2) of wheezing in the context of a URI over the preceding 12 months - 1 documented by a health care provider, and - 1 episode within the preceding 6 months 3. Either 2 episodes of (a), OR 2 episodes of (b), OR 1 episode of (a) AND 1 episode of (b) within the past 12 months: 1. Urgent care visit for wheezing which required treatment with at least a bronchodilator 2. Episode of wheezing which required treatment with oral corticosteroids

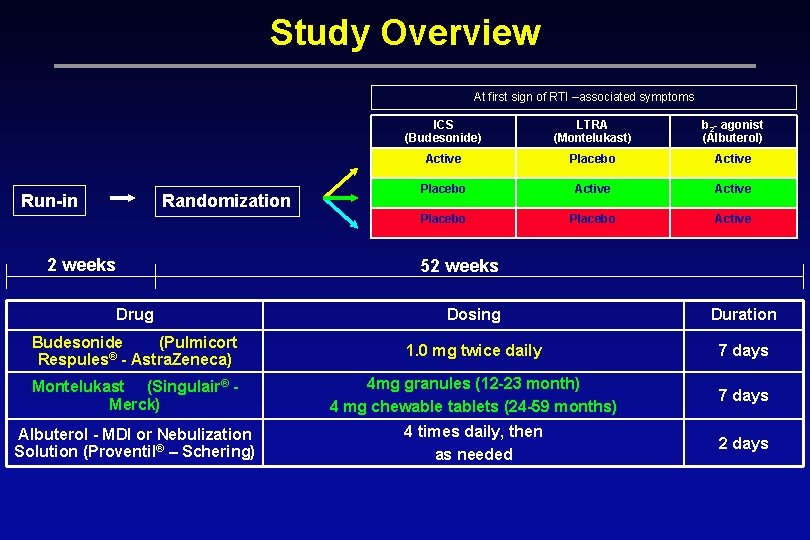
Study Overview At first sign of RTI –associated symptoms Run-in Randomization 2 weeks ICS (Budesonide) LTRA (Montelukast) b 2 - agonist (Albuterol) Active Placebo Active 52 weeks Drug Dosing Duration Budesonide (Pulmicort Respules® - Astra. Zeneca) 1. 0 mg twice daily 7 days Montelukast (Singulair® Merck) 4 mg granules (12 -23 month) 4 mg chewable tablets (24 -59 months) 7 days Albuterol - MDI or Nebulization Solution (Proventil® – Schering) 4 times daily, then as needed 2 days

Criteria for Starting Study Medications • Identification by parental interview of the typical pattern of symptom progression from respiratory illness to chest symptoms for each child • Development of an individualized action plan • Continual education to reinforce recognition of this symptom pattern • Initiation of a 7 -day study treatment course at the onset of the identified symptom pattern

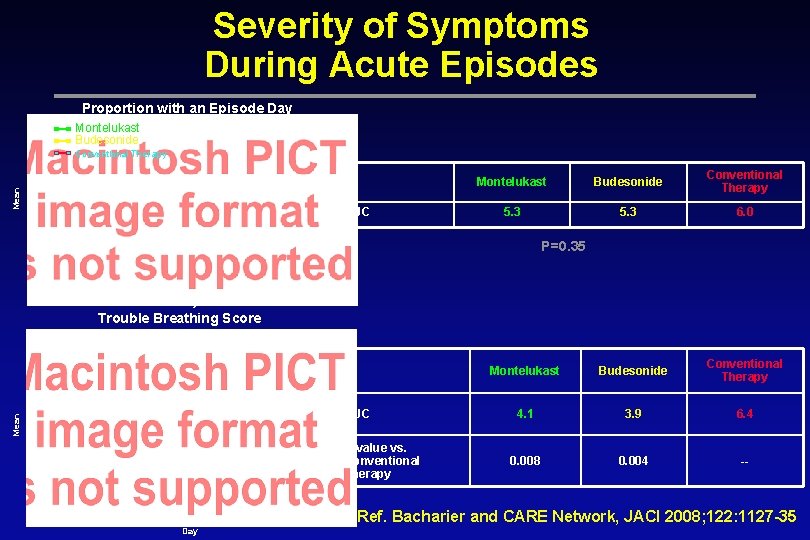
Severity of Symptoms During Acute Episodes Proportion with an Episode Day Montelukast Budesonide Mean Conventional Therapy AUC Montelukast Budesonide Conventional Therapy 5. 3 6. 0 P=0. 35 Day Trouble Breathing Score Montelukast Budesonide Conventional Therapy Mean AUC P-value vs. Conventional Therapy Montelukast Budesonide Conventional Therapy 4. 1 3. 9 6. 4 0. 008 0. 004 -- Ref. Bacharier and CARE Network, JACI 2008; 122: 1127 -35 Day

Summary – Effect of Treatment Over 12 Months in the Entire Study Group • The addition of montelukast or budesonide at the early signs of RTI-associated symptoms did not differ significantly from albuterol alone in: - The proportion of EFDs (primary outcome) - Oral corticosteroid use, urgent care visits, hospitalizations (secondary outcomes) - Growth or adverse effects (thus, both treatment approaches were safe)

Early Intervention Where do we go from here? • Intermittent ICS therapy? • Strategies to prevent asthma exacerbations? • Montelukast as initial therapy? • Anti-Ig. E? • SLIT? • Other immunomodulator therapy?

Maintenance vs Intermittent Inhaled Steroids In Wheezing Toddlers (MIST) Trial A trial in preschool children with recurrent wheezing, positive asthma predictive index and prior year severe wheezing exacerbation that compares the effect of maintenance low-dose ICS versus intermittent high-dose ICS at the onset of respiratory tract illnesses on the rate of exacerbations requiring systemic corticosteroids

MIST: Research Questions/Aims In preschool children with recurrent wheezing, +API, and severe exacerbation in prior year • Is maintenance low-dose ICS more effective than intermittent high-dose ICS with RTI on - Rate of exacerbations requiring systemic steroids? - Severity of acute RTI episodes? - Proportion of episode-free days? • Are there demographic, asthma/atopic or genetic predictors of treatment responses? • Are specific respiratory viruses associated with exacerbations and ICS response to treatment? • Are there treatment associated differential safety effects?

MIST: Hypothesis In preschool children with recurrent wheezing episodes, + API, and a severe wheezing episode in the year prior to enrollment, the rate of wheezing/asthma exacerbations requiring systemic corticosteroids is lower with (A) Maintenance daily low-dose ICS* Compared to (B) Intermittent high-dose ICS during RTI for 7 days* * Conventional therapy during RTI: albuterol qid x 48 hrs then prn

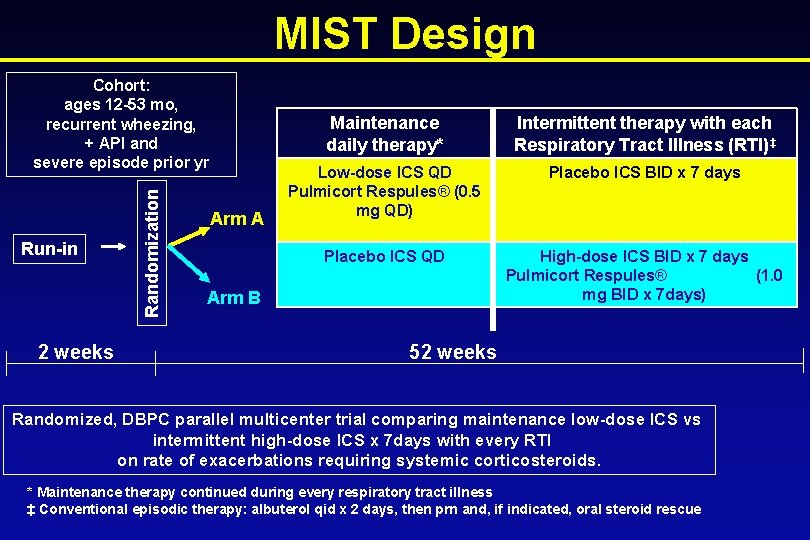
MIST Design Run-in 2 weeks Randomization Cohort: ages 12 -53 mo, recurrent wheezing, + API and severe episode prior yr Arm A Maintenance daily therapy* Intermittent therapy with each Respiratory Tract Illness (RTI)‡ Low-dose ICS QD Pulmicort Respules® (0. 5 mg QD) Placebo ICS BID x 7 days Placebo ICS QD High-dose ICS BID x 7 days Pulmicort Respules® (1. 0 mg BID x 7 days) Arm B 52 weeks Randomized, DBPC parallel multicenter trial comparing maintenance low-dose ICS vs intermittent high-dose ICS x 7 days with every RTI on rate of exacerbations requiring systemic corticosteroids. * Maintenance therapy continued during every respiratory tract illness ‡ Conventional episodic therapy: albuterol qid x 2 days, then prn and, if indicated, oral steroid rescue

Primary Efficacy Outcome • Rate of severe exacerbations requiring systemic corticosteroids.

Secondary Efficacy Outcomes • Time to 1 st and 2 nd severe exacerbation requiring systemic steroids overall • Rate of and time to 1 st and 2 nd severe exacerbation during RTI requiring systemic steroids • Time to treatment failure • Urgent care visits/ED visits/hospitalizations • Absences (school/daycare/work) related to wheezing

Secondary Efficacy Outcomes (II) • Severity of symptoms during acute RTI • Episode-free days • Albuterol use (number of actuations or nebs) • Quality of life (Peds-QL generic and asthma specific) • e. NO levels

Safety Outcomes • Steroid adverse effects - Growth: height and weight - Thrush - Hoarseness • SAEs • AEs

Pediatric Asthma: Early Intervention • Variability in asthma control should be anticipated • Reasons for variability should be identified and addressed • Inhaled glucocorticoids are the preferred first line long-term control therapy • Inhaled glucocorticoids are effective in alleviating symptoms but do not alter the natural history of asthma. • Alternative therapies merit investigation as potential interventions following early diagnosis of asthma.

Ref. Journal of Allergy and Clinical Immunology 2007; 120: 1043 -1050



Immediate Practice Changes • Record information in EMR related to the natural history of asthma. • Flag risk factors in EMR for the development of asthma. • Incorporate management techniques to control symptoms of asthma and prevent exacerbations.

Asthma Management Individualized or “Personalized” Approach • Utilize asthma characteristics, biomarkers, and genetics to “profile” asthma severity and prognosis. • Select medications based on driving factors of disease presentation and predictors of response. • Monitor response and assess reasons for treatment failure. • Develop proactive approach and adjust therapy accordingly.

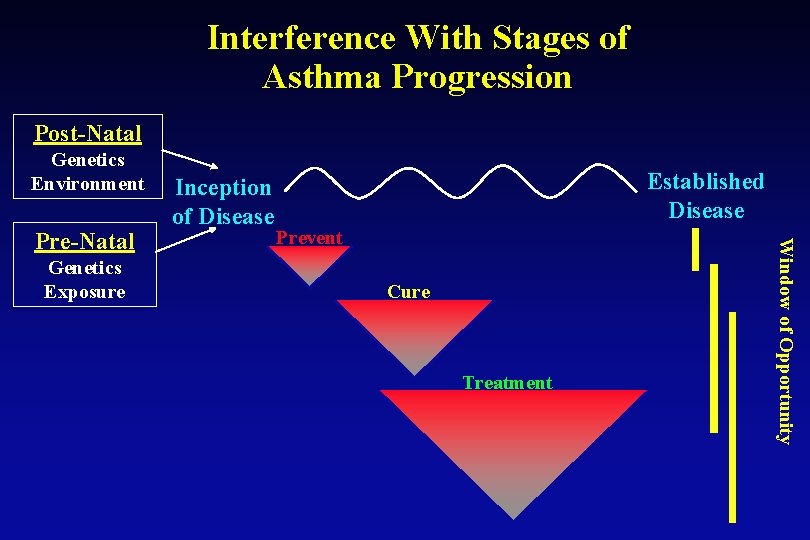
Interference With Stages of Asthma Progression Post-Natal Genetics Environment Genetics Exposure Established Disease Prevent Cure Treatment Window of Opportunity Pre-Natal Inception of Disease
 Pathology of asthma
Pathology of asthma Classification of asthma
Classification of asthma Asthma treatment
Asthma treatment Asthma exacerbation treatment
Asthma exacerbation treatment What is life
What is life Phonemic awareness in young children
Phonemic awareness in young children Hampshire early years advisory team
Hampshire early years advisory team Working with young children/answer key chapter 1
Working with young children/answer key chapter 1 Unit 10 caring for children and young people
Unit 10 caring for children and young people Trauma symptom checklist for children™ screening form
Trauma symptom checklist for children™ screening form Alcohol consumption causes blood vessels to contract.
Alcohol consumption causes blood vessels to contract. Asthma diagnosis criteria
Asthma diagnosis criteria Asthma pathophysiology
Asthma pathophysiology Allergy asthma immunol res impact factor
Allergy asthma immunol res impact factor Asthma progression
Asthma progression Asthma
Asthma Gina guidelines for asthma
Gina guidelines for asthma Global initiative for asthma
Global initiative for asthma Laba asthma
Laba asthma Asthma oshit
Asthma oshit Aerochamber definition
Aerochamber definition Asthma attack
Asthma attack Cause of asthma
Cause of asthma Ami core measures
Ami core measures Allergic rhinitis icd10
Allergic rhinitis icd10 International severe asthma registry
International severe asthma registry Asthma
Asthma Saba and laba examples
Saba and laba examples Monday dyspnea
Monday dyspnea Asthma pathophysiology
Asthma pathophysiology Asthma index
Asthma index Asthma pocket guide
Asthma pocket guide Alveoli are most similar to brainpop
Alveoli are most similar to brainpop Asthma vs copd spirometry
Asthma vs copd spirometry Asthma stufenplan
Asthma stufenplan Objective data for asthma
Objective data for asthma Spirometry
Spirometry Dr arshad ejazi
Dr arshad ejazi Mild moderate severe asthma exacerbation
Mild moderate severe asthma exacerbation Driver diagram asthma
Driver diagram asthma Ivet institute
Ivet institute Asthma types
Asthma types Asthma symptoma
Asthma symptoma